Published online Apr 7, 2007. doi: 10.3748/wjg.v13.i13.1893
Revised: March 7, 2006
Accepted: March 21, 2007
Published online: April 7, 2007
Inflammatory bowel diseases (IBD) are chronic inflammatory conditions of the gastrointestinal tract, which clinically present as one of two disorders, Crohn’s disease or ulcerative colitis. Mainstays of drug treatments for IBD include aminosalicylates, corticosteroids and immunosuppressants such as azathioprine, methotrexate and cyclosporin. Advances in basic research of the pathophysiological process in IBD have been applied to generate a variety of new therapeutics targeting at different levels of the inflammatory processes. New therapies are classified as: (1) Anti-TNFα antibodies; (2) Recombinant cytokines; (3) Selective adhesion blockade; (4) Growth factors; (5) Innate immunostimulation; (6) Nucleic acid based therapies; (7) Gene therapy; (8) Autologous bone-marrow transplantation; (9) Helminths and (10) Extracorporeal immunomodulation. All treatments have the potential to provide more effective and safe treatment for IBD.
- Citation: Yamamoto-Furusho JK. Innovative therapeutics for inflammatory bowel disease. World J Gastroenterol 2007; 13(13): 1893-1896
- URL: https://www.wjgnet.com/1007-9327/full/v13/i13/1893.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i13.1893
Inflammatory bowel diseases (IBD) including ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory conditions affecting the gastrointestinal tract. The etiopathogenesis has not been fully elucidated but is currently presumed to result from a complex interplay among genetic, environmental, microbial and immune factors[1].
The current state of therapy for patients with IBD is not very satisfactory. In patients with active CD, an adequate course of corticosteroids induces remissions in only 60% to 70% and in this group with response, many patients relapse during tapering of the steroids dose[2]. About 33% to 50% of patients with fulminant ulcerative colitis who are admitted to the hospital have their colon removed during the same admission because of failure of conventional medical therapy[3].
Patients with IBD frequently experience relapse and traditional medical treatments are not potent enough to keep in remission for long-term periods.
Biological approach to IBD therapy has developed in recent years as a result of understanding of the mucosal immunology processes in intestinal inflammation. The first effective biological therapy approved for commercial use is infliximab, a chimeric monoclonal antibody directed against tumour necrosis factor α (TNFα), for the treatment of CD in 1998 and UC in 2006 by the FDA. Infliximab is indicated for induction and maintenance of remission in patients with active luminal inflammatory CD refractory to conventional therapy, those with draining enterocutaneous and perianal fistulas and recently, for patients with moderate to severe active UC despite treatment with concurrent medications. However, a meta-analysis of 34 studies (896 patients with UC) showed that long-term remission (9 mo) was 39%[4].
Conventional treatment including infliximab can provide clinical benefit, reduce signs and symptoms of disease, and improve quality of life, however, most do not significantly alter the long-term course of the disease or the underlying immunopathology.
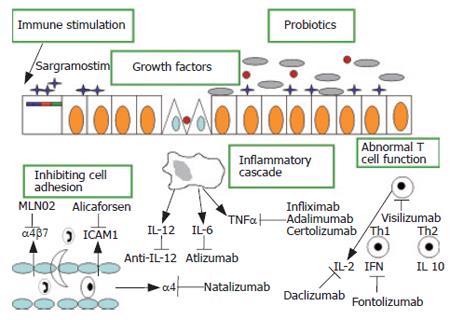
New therapies, which are targeted at specific disease mechanisms, have the potential to provide more effective and safe treatments for human diseases as shown in Figure 1.
There are several categories of treatments that are relevant to IBD, including (1) New anti-TNFα antibodies; (2) Recombinant cytokines; (3) Selective adhesion blockade; (4) Growth factors; (5) Immunostimulation; (6) Nucleic acid based therapies; (7) Gene therapy; (8) Autologous bone-marrow transplantation; (9) Helminths, (10) Extracorporeal immunomodulation. A number of new anti-TNFα agents are currently being investigated in phase III studies in CD, including a pegylated humanized anti-TNFα fragment, CDP870 (certolizumab) and a fully human anti-TNFα antibody (Adalimumab) that binds with a high affinity and specificity to human soluble TNFα.
An initial randomized controlled trial (RCT) of certolizumab in active CD demonstrated a significant benefit in achieving remission, through not response[5]. Another trial demonstrated a benefit in a subgroup of CD patients with elevated CRP (10 mg per litre) and found a statistically significant response in the highest dose tested[6].
Recently, CHARM and CLASSIC II trials demon-strated the efficacy and safety of adalimumab in the induction and maintenance of clinical remission in moderate to severe CD for up to 56 wk[7,8]. An important aspect of anti-TNFα agents is the development of anti-idiotype antibodies directed towards the murine portion of the chimeric antibody resulting in hypersensitivity and decreased responsiveness. On the other hand, adalimumab is a humanized monoclonal agent to which the sensitization and development of anti-idiotype antibodies are much less than infliximab.
A second approach has been to block steps in T-cell development by targeting cytokines involved in T-cell differentiation and activation such as IL-12. A recent RCT of 79 patients with CD receiving 1 mg or 3 mg of anti-IL-12 monoclonal antibody versus placebo demonstrated a response in 75% of CD patients compared with 25% in the placebo group[9]. Other antibodies have been generated against T-cell subsets including CD3+ cells (visilizumab) and CD25+ cells (daclizumab and basiliximab) for UC. Pilot studies have shown promising results in steroid-resistant UC patients[10].
IL-6 participates in a variety of critical functions, including T cell growth and differentiation, as well as B-cell proliferation. A pilot study showed in patients with active CD treated with an antibody directed against the IL-6 receptor (Atlizumab), 80% responded at the full dose compared with 31% in the placebo group[11].
RDP58 is an oral decapeptide that decreases the activity of TNFα, IL-2, IL-12 and IFN-γ. Phase II studies showed high remission rates and minimal toxicity in UC patients[12]. Most of the strategies have targeted to interrupt cytokine activity through inhibition of pathways promoting cytokine production, or by directly blocking cytokine action.
A different approach aims to block leukocyte migration to sites of inflammation by interfering with cell adhesion molecules. There are three targeting therapies in clinical trials to date including natalizumab (integrin α4 subunit) and MLN-02 (integrin α4β7). An RCT in 248 patients with moderate to severe CD randomly assigned to receive natalizumab or placebo, demonstrated high rates of clinical remission and response, and improved quality of life and C-reactive protein levels[13]. Studies of MLN-02 have demonstrated equivocal results in CD[14] but promising effects in UC, with responses and remission rates of 66% and 33% compared with 33% and 15%, respectively, in those receiving placebo[15].
Augmenting intestinal repair could be another approach for patients with IBD. Topical administration of epidermal growth factor (EGF) seemed to be effective in promoting both initial response and long-term maintenance in 20 patients with left-sided UC[16].
Growth hormone and a high-protein diet resulted in a significant decrease in Crohn´s disease activity index (CDAI) in the active treatment group as compared with placebo group[17]. This effect is attributed to growth hormone because it might enhance the intestinal barrier function, including decreasing intestinal permeability and increasing intestinal protein turnover as well as has stimulatory effects on neutrophil function.
Recent evidence has shown the importance of innate immune mechanisms in sustaining homeostasis in the gastrointestinal mucosa and some defects in these intestinal innate immune responses in patients with CD[18]. Some reports suggest that agents that act to enhance innate immune defenses can indeed confer therapeutic benefit. Two agents (G-CSF or filgastrim and GM-CSF sargramostin) have been evaluated in patients with CD. The endogenous growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF) performs important functions in both the phagocytic and epithelial components of intestinal early innate immune defense. GM-CSF is expressed by both CD4+ T cells and Paneth cells in the intestine, and its receptors have been recently demonstrated to be present on intestinal epithelial cells which proliferate in response to GM-CSF in vitro[19,20]. Both pilot studies suggested a benefit[21,22], though GM-CSF appeared more effective. A recent RCT of 124 patients found a significant benefit in response at 100-point decrease in CDAI and in remission. The response was sustained for a mean of 8-10 wk after discontinuation of therapy[23].
Nucleic acid based therapies have focused on the use of antisense phosphorothioate oligonucleotide to the p65 subunit of NF-κB and ICAM-1 antisense oligonucleotide (Alicaforsen, ISIS-2302). In a pilot study 11 steroid-refractory or resistant IBD patients were given a single dose of rectal antisense NF-κB p65 oligonucleotide. An improvement in clinical, endoscopic and histologic scores was seen at d 7 in 71% of the treatment group compared with 25% of the placebo group[24].
ISIS-2302 is a 20-base pair complementary nucleotide chain which hybridizes with ICAM-1 mRNA and is thus degraded by RNAse-H and the message and expression of ICAM-1 is therefore decreased. In patients treated with doses between 300-350 mg infused 3 times per week for 4 wk, it seemed to have higher benefit in those with active CD[25].
Gene therapy strategies using plasmid IL-10 vectors or an adenovirus IL-10 construct seem to be a potent approach for the treatment of IBD. Barbara et al[26] reported that gene transfer was achieved by intraperitoneal injection of non-replicating human adenovirus bearing IL-10 gene, either 24 h before or 1 h after intrarectal administration of trinitrobenzenesulfonic acid (TNBS) in rats. IL-10 gene transfer prior to colitis improved colitis macroscopically and histologically.
Allogenic bone-marrow transplants in CD patients were noted to induce prolonged disease remission, providing evidence of the role of bone-marrow T cells in this disease.
The goal of autologous haematopoietic stem-cell transplantation (HSCT) is resetting T cell responses by eliminating all circulating T cells. A phase I study in 12 patients with refractory CD showed that 11 patients entered a sustained remission (CDAI < 150). After a median follow-up of 18.5 mo, only one patient has developed a recurrence of active CD, which occurred 15 mo after HSCT[27].
Another strategy for resetting the T-cell repertoire has been proposed based on the helminths. The immune system has evolved with the presence of these helminthes, which function to expand the T regulatory cell population, enhancing IL-10 production and shifting a TH1 type process more towards TH2[28]. An RCT of using 2500 Trichiuris suis ova, a pig worm, administered orally once every two weeks, in UC has shown a response of 44% of the treatment group versus 14% of receiving placebo[29]. An open label study of 29 patients with active CD identified high rates of response (79.3%) and remission (72.4%)[30].
Early reports on the benefit of lymphocyte apheresis to treat CD described selective extracorporeal T cell depletion by ultracentrifugation in patients with active CD, the majority of whom went into long-lasting remission and decreased steroid use to zero. Two strategies that decrease specific lymphocyte subpopulations were developed in Japan. One technique is a leukocyte removal filter column-CellsorbaTM. All leukocyte subpopulations, 99% of granulocytes and macrophages and 40%-60% of lymphocytes as well as platelets, are trapped within the filter made of polyester fibers. In patients with CD an intensive induction protocol-leukocytopheresis every week for 5 wk induced remission in 50% of the patients. An alternative leukocytopheresis method, Adacolumn, uses a column adsorbing granulocytes and monocytes/macrophages to 2 mm in diameter of cellulose acetate beads, without significantly adsorbing lymphocytes. Several trials demonstrated a positive effect of the column in active UC[31,32].
Immunostimulatory DNA sequences, ISS-DNA, also known as CpG DNA, are unmethylated CpG dinucleotides within consensus sequences present in bacterial and viral genomes. ISS-DNA and their synthetic analogues activate innate immunity via Toll-like receptor 9. Liposomal immunostimulatory DNA sequence (ISS-ODN) was shown to prevent and ameliorate the severity of colitis in animal models[33] and therefore may be effective also in the treatment of human IBD. Clinical trials to test their efficacies are underway.
Currently, numerous innovative agents for IBD have been available for the treatment of patients with IBD refractory to conventional therapy including infliximab. In the near future, the enticing concept of combining different therapeutic approaches targets at different components of the inflammatory process. Intensive basic research continues in order to generate new targets or novel approaches that intervene at other steps in the pathophysiological processes.
S- Editor Zhu LH L- Editor Zhu LH E- Editor Zhou T
| 1. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2747] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 2. | Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 431] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Travis SP, Farrant JM, Ricketts C, Nolan DJ, Mortensen NM, Kettlewell MG, Jewell DP. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 518] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 4. | Gisbert JP, González-Lama Y, Maté J. Systematic review: Infliximab therapy in ulcerative colitis. Aliment Pharmacol Ther. 2007;25:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Winter TA, Wright J, Ghosh S, Jahnsen J, Innes A, Round P. Intravenous CDP870, a PEGylated Fab' fragment of a humanized antitumour necrosis factor antibody, in patients with moderate-to-severe Crohn's disease: an exploratory study. Aliment Pharmacol Ther. 2004;20:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OØ, Innes A. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 7. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1620] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 8. | Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, Panaccione R, Wolf D, Kent JD, Bittle B. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56:1232-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 771] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 9. | Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 614] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 10. | Creed TJ, Probert CS, Norman MN, Moorghen M, Shepherd NA, Hearing SD, Dayan CM. Basiliximab for the treatment of steroid-resistant ulcerative colitis: further experience in moderate and severe disease. Aliment Pharmacol Ther. 2006;23:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn's disease. Gastroenterology. 2004;126:989-996; discussion 947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 471] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 12. | Travis S, Yap LM, Hawkey C, Warren B, Lazarov M, Fong T, Tesi RJ. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm Bowel Dis. 2005;11:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnálek P, Zádorová Z, Palmer T, Donoghue S. Natalizumab for active Crohn's disease. N Engl J Med. 2003;348:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 617] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 14. | Feagan B, Greenberg G, Wild G. Efficacy and safety of a humanized alpha4beta7 antibody in active Crohn's disease. Gastroenterology. 2003;124:A25. [DOI] [Full Text] |
| 15. | Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 530] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 16. | Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Slonim AE, Bulone L, Damore MB, Goldberg T, Wingertzahn MA, McKinley MJ. A preliminary study of growth hormone therapy for Crohn's disease. N Engl J Med. 2000;342:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Yamamoto-Furusho JK, Korzenik JR. Crohn's disease: innate immunodeficiency. World J Gastroenterol. 2006;12:6751-6755. [PubMed] |
| 19. | Fukuzawa H, Sawada M, Kayahara T, Morita-Fujisawa Y, Suzuki K, Seno H, Takaishi S, Chiba T. Identification of GM-CSF in Paneth cells using single-cell RT-PCR. Biochem Biophys Res Commun. 2003;312:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Ramsay RG, Micallef SJ, Williams B, Lightowler S, Vincan E, Heath JK, Mantamadiotis T, Bertoncello I. Colony-stimulating factor-1 promotes clonogenic growth of normal murine colonic crypt epithelial cells in vitro. J Interferon Cytokine Res. 2004;24:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Dieckgraefe BK, Korzenik JR. Treatment of active Crohn's disease with recombinant human granulocyte-macrophage colony-stimulating factor. Lancet. 2002;360:1478-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Korzenik JR, Dieckgraefe BK. An open-labelled study of granulocyte colony-stimulating factor in the treatment of active Crohn's disease. Aliment Pharmacol Ther. 2005;21:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Korzenik JR, Dieckgraefe BK, Valentine JF, Hausman DF, Gilbert MJ. Sargramostim for active Crohn's disease. N Engl J Med. 2005;352:2193-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 24. | Loftberg R, Neurath M, Ost A, Petterson S. Topical NF-κB p65 antisense oligonucleotides in patients with active distal colonic IBD. A randomized, controlled pilot trial. Gastroenterology. 2002;122:A60. |
| 25. | Barish CF. Alicaforsen therapy in inflammatory bowel disease. Expert Opin Biol Ther. 2005;5:1387-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Barbara G, Xing Z, Hogaboam CM, Gauldie J, Collins SM. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut. 2000;46:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Doetze A, Satoguina J, Burchard G, Rau T, Löliger C, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 2000;12:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 263] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Summers RW, Elliott DE, Urban JF, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 525] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 30. | Summers RW, Elliott DE, Urban JF, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 529] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 31. | Kohgo Y, Ashida T, Maemoto A, Ayabe T. Leukocytapheresis for treatment of IBD. J Gastroenterol. 2003;38 Suppl 15:51-54. [PubMed] |
| 32. | Hanai H. Positions of selective leukocytapheresis in the medical therapy of ulcerative colitis. World J Gastroenterol. 2006;12:7568-7577. [PubMed] |
| 33. | Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 595] [Article Influence: 28.3] [Reference Citation Analysis (0)] |