Published online Mar 28, 2007. doi: 10.3748/wjg.v13.i12.1811
Revised: December 3, 2006
Accepted: January 25, 2007
Published online: March 28, 2007
AIM: To report the clinicopathological features and magnetic resonance imaging (MRI) findings of solid and pseudopapillary tumor (SPT) of pancreas.
METHODS: From 1981 to 2005, 26 surgically treated cases of SPT were retrospectively reviewed. MRI findings of the latest 11 consecutive SPT cases were investigated.
RESULTS: There were 25 women and one man having SPT (median age: 23 year) with a median tumor size of 7.5 cm. Among them, nine patients developed solid pseudopapillary carcinoma. During the median follow-up period of 66 mo, the 5-year survival rate of the 26 SPT patients was 96.2%. Three MRI features were proposed including Type 1 image, displaying SPT with completely solid part. All SPT patients with type 1 image were detected incidentally. Type 2 image displays of SPT with solid mass hemorrhage and type 3 image with massive hemorrhage. All the eight SPT patients with type 2 and 3 images suffered abdominal pain due to hemorrhage from SPT.
CONCLUSION: SPT had a favorable survival rate irrespective of surgical procedures, malignancy, and MRI findings, however, MRI could reliably correlate with its clinicopathological features.
- Citation: Yu CC, Tseng JH, Yeh CN, Hwang TL, Jan YY. Clinicopathological study of solid and pseudopapillary tumor of pancreas: Emphasis on magnetic resonance imaging findings. World J Gastroenterol 2007; 13(12): 1811-1815
- URL: https://www.wjgnet.com/1007-9327/full/v13/i12/1811.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i12.1811
Solid and pseudopapillary tumor (SPT) of pancreas is a rare disease entity, occurring mainly in young women[1-4]. The pathologic features of SPT are well characterized[1,2,5]. Since 1959, over 500 cases of SPT of the pancreas have been reported in literature[3,6]. According to the World Health Organization’s (WHO) classification, it is designated as tumors of the exocrine pancreas[1], It is also well known for its indolent biologic behavior. Despite its low malignant potential, proximately 15% of patients with SPT develop metastatic disease, most involving the liver or peritoneum[2]. Even in the presence of disseminated disease, the clinical course is usually favorable[1-3].
With recent advances in diagnostic modalities, including ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and fine-needle aspiration biopsy (FNAB), the tumor was more frequently reported[1,3,7,8]. CT may help to differentiate between SPTs and pancreatic cystic neoplasm, whereas MRI may help to differentiate those from islet cell tumors[7].
Here, we reviewed 26 cases of SPT treated at Chang Gung Memorial Hospital from 1981 to 2005 with respect of clinicopathological, radiological, operative findings, and outcomes. With emphasis on MRI findings, we proposed three specific MRI features to correlate this rare disease entity with its clinicopathological features more precisely.
From 1981 to 2005, 26 consecutive patients with SPT of the pancreas underwent surgical treatment at the Department of Surgery, Chang Gung Memorial Hospital, Taipei, Taiwan. Six of these 26 patients had been reported previously[9]. 20 new patients with SPT were included in this study.
Preoperative evaluation of SPT included history taking physical examinations, routine laboratory examinations, ultrasonography (US), endoscopic ultrasonography (EUS), abdominal computed tomography (CT), and magnetic resonance imaging (MRI). The imaging features of the latest 11 consecutive SPT cases underwent MRI study by one of the authors (Tseng JH) (Magnetom Vision; Siemens Erlangen, Germany; projection technique and multislice plus maximum intensity projection) were studied. Of the 26 SPT patients discharged from the hospital after surgical treatment, all of the patients were closely followed up at regular intervals until death or until the time of this manuscript writing.
Survival was calculated and plotted using the Kaplan-Meier method from the time of surgical treatment until the writing of this manuscript. All statistical analyses were performed utilizing the SPSS computer software (Version 12.0, Chicago, IL, USA).
Table 1 summarizes the clinicopathological features of the 26 SPT patients. There were 25 females and 1 male ranging in ages from13 to 57 (median/mean, 23.0/25.2) yrs. Abdominal pain comprises the most common symptom for SPT (38.5%). Five (19.2%) of the 26 SPT patients were detected incidentally during physical check-up. Two of 26 SPT patients developed jaundice (7.7%). The size of SPT ranged from 3.8 to 15.0 (median, 7.5) cm. All known tumor markers including CEA and CA 19-9 are within normal limit, irrespective of malignancy. Pancreatic head is the most frequent location of tumor (Table 1). Two of the 26 SPT patients developed into unresectable status due to multiple liver metastasis and carcinomatosis. Among 26 SPT patients, nine patients were classified as solid pseudopapillary carcinoma by the World Health Organization’s (WHO) classification (Table 2). Four of nine solid pseudopapillary carcinoma developed duodenal invasions, while the other three developed portal vein invasions. The duration of follow-up ranged from 10.1 to 236.8 mo (median, 66.0) mo. During the follow-up period, 2 patients of 9 malignant SPT (22.2%) had distant metastasis of SPT after surgical treatment. Two of the 26 patients died of causes unrelated to the tumor or surgical procedure. One underwent Whipple’s operation and died of hepatic encephalopathy after a grand mal seizure. The other one underwent bypass surgery and died of hepatic failure due to hepatitis exacerbation. All the other cases have survived until now, whether cases were benign or malignancy. The five-year survival rate of the 26 SPT patients was 96.2%.
| n | (%) | |
| Gender | ||
| Male | 1 | 3.8 |
| Female | 25 | 96.2 |
| Symptom | ||
| No symptom | 5 | 19.2 |
| Abdominal pain | 10 | 38.5 |
| Abdominal palpable mass | 5 | 19.2 |
| Jaundice | 2 | 7.7 |
| Epigastralgia | 4 | 15.4 |
| Tumor marker | ||
| CEA < 5 (μg/L) | 26 | 100 |
| CA 19-9 < 37 (kU/L) | 26 | 100 |
| Tumor location | ||
| Head | 11 | 42.3 |
| Body | 3 | 11.6 |
| Tail | 6 | 23.1 |
| neck and body | 1 | 3.8 |
| body and tail | 5 | 19.2 |
| Resectability | ||
| Resectable | 24 | 92.3 |
| Unresectable | 2 | 7.7 |
| Operation method | ||
| Whipple’s operation | 8 | 30.8 |
| Partial pancreatectomy | 3 | 11.6 |
| Distal pancreatectomy | 7 | 26.9 |
| Enucleation of the pancreatic tumor | 6 | 23.1 |
| Bypass surgery | 1 | 3.8 |
| Biopsy only | 1 | 3.8 |
| Tumor behavior | ||
| Solid-pseudopapillary neoplasm | 17 | 56.4 |
| Solid-pseudopapillary carcinoma | 9 | 34.6 |
| No. | Age(yr)/Sex | Tumor location | Extension | Vascular invasion | Operation method | Metastasis | Metastasis(mo) | Status | Follow-up(mo) |
| 1 | 36/F | Head | Duodenal wall | - | Whipple’s operation | - | - | Alive | 70 |
| 21 | 34/F | Tail | - | - | Distal pancreatectomy | - | - | Alive | 12 |
| 3 | 39/F | Head | - | - | Whipple’s operation | Liver | 39 | Alive | 58 |
| 4 | 22/F | Neck, | - | PV | Partial pancreatectomy, | - | - | Alive | 202 |
| body | |||||||||
| 5 | 18/F | Head | Duodenal wall | - | Whipple’s operation | - | - | Alive | 145 |
| 6 | 16/F | Head | Duodenal wall | - | Whipple’s operation | - | - | Alive | 56 |
| 7 | 20/F | Body, | Extrapancreatic | - | Biopsy only | Liver, omentum | 0 | Alive | 75 |
| tail | Peritoneum | ||||||||
| Pelvic wall | |||||||||
| 8 | 28/F | Head | - | PV | Whipple’s operation | - | - | DOC | 66 |
| 9 | 19/F | Head | - | PV, SMA | Roux-en-Y cystojejunostomy | - | - | DOC | 83 |
Table 3 summarized the MRI features. MRI was used in eleven SPT patients with three specific features correlating well with pathological features. MRI had three specific features correlating well with pathological features. Three patients had type 1 image and all the three patients had incidentally found SPT.
| No. | Type of image | Presentation | Age | G | Size(cm) | Location | T1WI | Enhancement | T2WI | H |
| 1 | I | RUQ mass, incidentally noted | 28 | F | 3.5 | Tail | Hypointense homogenously | Gradual enhancement of solid component | Hypointense homogenously | (-) |
| 2 | I | GI upset, incidentally noted | 22 | F | 7.5 | Body and tail | Hypointense homogenously | Gradual enhancement of solid component | Hypointense homogenously | (-) |
| 3 | I | RUQ mass, incidentally noted | 22 | F | 6 | Head | Hypointense homogenously | Gradual enhancement of solid component | Mild hyperintense | (-) |
| 4 | II | Abdominal pain | 14 | F | 5 | Body | Hyperintense heterogeneously | Heterogenous | Hyperintense (hyperintense areas on T1WI ) | (+) |
| 5 | II | Abdominal pain | 29 | F | 5 | Head | Hyperintense heterogeneously | Heterogenous | Hyperintense (hyperintense areas on T1WI ) | (+) |
| 6 | II | Abdominal pain | 33 | F | 11 | Tail | Hyperintense heterogeneously | Heterogenous | Hyperintense (hyperintense areas on T1WI ) | (+) |
| 7 | II | Abdominal pain | 29 | F | 7 | Head | Hyperintense heterogeneously | Heterogenous | Hyperintense (hyperintense areas on T1WI ) | (+) |
| 8 | II | Abdominal pain | 23 | F | 7.6 | Head | Hyperintense heterogeneously | Heterogenous | Hyperintense (hyperintense areas on T1WI ) | (+) |
| 9 | III | Abdominal pain | 27 | F | 6 | Tail | Hypo and hyperintense homogenously | Capsular enhancement, Gradual enhancement of solid compnent | Hyperintense (hyperintense areas on T1WI ) | (+) |
| 10 | III | Abdominal pain | 36 | F | 7 | Head | Hyperintense heterogeneously | Heterogenous | Hyperintense (hyperintense areas on T1WI ) | (+) |
| 11 | III | Abdominal pain | 16 | F | 5 | Head | Hyperintense heterogeneously | Heterogenous | Hyperintense (hyperintense areas on T1WI ) | (+) |
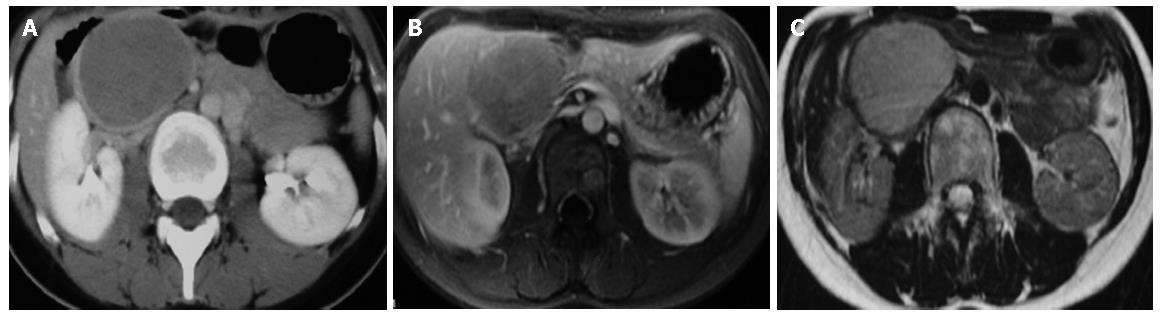
Type 1 image means SPT with completely solid part. T1W1 image revealed homogenously hypointense and slightly hyperintense than pancreas parenchyma on T2W1 image. Strong and rapid enhancement and gradually fading pattern could be observed (Figure 1).
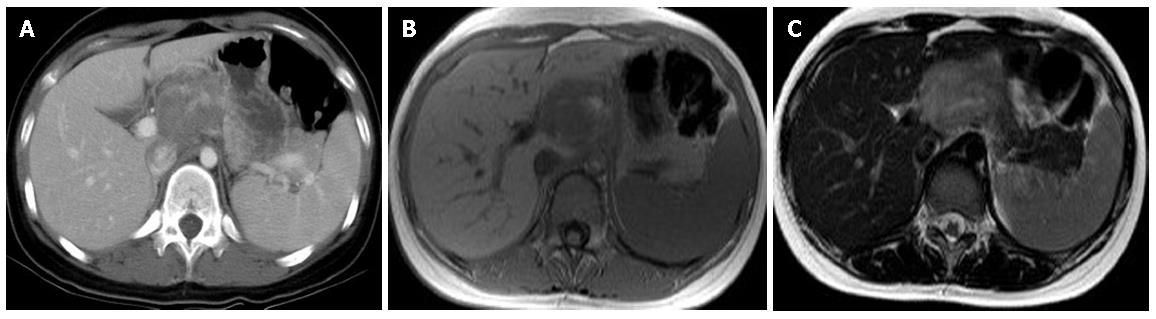
Type 2 image means SPT with solid mass with hemorrhage. T1W1 image revealed hypointense with heterogeneously hyperintense area. The hyperintense areas on T1W1 appeared slightly hyperintense on T2W1 image meaning hemorrhage while enhancement of capsule and gradual enhancement of solid part (Figure 2).
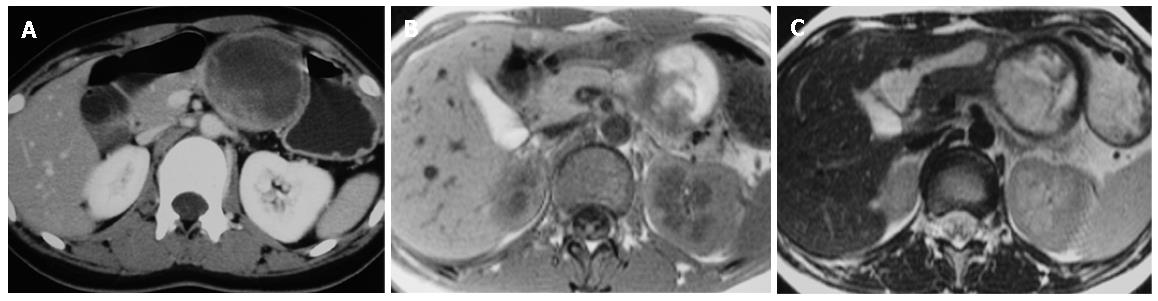
Type 3 image means SPT with massive hemorrhage. T1W1 revealed mainly hyperintense with intermediate and hypointense areas. The hyperintense areas on T1W1 image appeared slightly hyperintense on T2W1 image. Only capsular enhancement could be detected (Figure 3). All the eight SPT patients with type 2 and 3 images suffered abdominal pain due to hemorrhage from SPT.
As shown in our results, solid pseudopapillary tumor (SPT) of pancreas predominated in adolescent girls and young women (median/mean: 23.0/25.2 years; rang 13-57 years) and is uncommon in men (1/26; 3.8%). Although SPT of pancreas is uncommon, it has been recognized with increasing frequency in recent years, accounting for approximately 1%-2% of all exocrine pancreatic tumors[1,3]. Although literature showed no preferential localization within the pancreas, the head of pancreas is the preferential site of the occurrence of SPT in this study with an incidence rate of 42.3%[1]. The etiology and pathogenesis of SPT is unknown[1,3,6]. Very few women developed a SPT after long-term use of hormone contraceptives[1]. The striking sex and age distribution point to genetic and hormonal factors and our previous report indicated an association with endocrine disturbances including overproduction of progesterone[1,6]. Usually, the neoplasm caused abdominal discomfort and pain (10/26; 38.5%) or they were found incidentally on routine physical examination (5/26; 19.2%). As shown in this study, jaundice is rare, even in tumor that originated from the head of the pancreas, and there is no association with endocrine function. All known tumor markers including CEA and CA 19-9 are within normal limit, irrespective of malignancy.
Regarding the role of endoscopic ultrasound (EUS) in evaluation of SPTs, Lee proposed that the characteristic sonographic findings of solid and papillary epithelia neoplasm were well-encapsulated, cystic and solid masses, but sometimes the mass was seen as a pure solid-looking mass or had internal septations or calcification[10]. Fine-needle aspiration (FNA) may have played an important role in preoperative planning by helping distinguish SPTs from other pancreatic lesions with a significantly different prognosis and treatment[11]. EUS-FNA represents a diagnostic technique commonly used in adults that may be useful in identifying the rare pediatric patient with pancreatic malignancy[12]. Thus, accurate diagnosis of the special type of pancreatic tumor is obviously important. Fine-needle aspiration biopsy and cytologic analysis or excisional biopsy and histologic analysis are sometimes needed for definitive diagnosis.
Regarding radiological study, CT may help to differentiate SPT from other cystic neoplasm whereas MRI may help to differentiate SPT from islet cell tumors[7]. Regarding radiological features of SPT, MRI revealed an encapsulated mass lesion with solid and cystic component as well as hemorrhage without obvious internal septation should arouse the suspicion of SPT of pancreas, especially in a young female patient. As shown in this study, MRI may be superior to CT in terms of correlation between radiological and clinicopathlogical findings of SPT. Furthermore, in the setting of renal insufficiency or dye allergy when contrast is contraindicated, MRI had an advantage when compared with CT. We specifically proposed three MRI features to correlate well with clinicopathological features. Type 1 image means SPT with completely solid part. T1W1 image revealed homogenously hypointense and slightly hyperintense than pancreas parenchyma on T2W1 image. Strong and rapid enhancement and gradually fading pattern could be observed. Type 2 image means SPT with solid mass with hemorrhage. T1W1 image revealed hypointense with heterogeneously hyperintense area. The hyperintense areas on T1W1 appeared slightly hyperintense on T2W1 image, meaning hemorrhage, while enhancement of capsule and gradual enhancement of sold part. Type 3 image means SPT with massive hemorrhage. T1W1 revealed mainly hyperintense with intermediate and hypointense areas. The hyperintense areas on T1W1 image appeared slightly hyperintense on T2W1 image. Only capsular enhancement could be detected.
As shown in this study, a few SPT could be found to be attached to the pancreas or even an extrapancreatic locations. Invasion of the adjacent organs or the portal vein is not uncommon. As shown in this series, few metastasizing SPTs (2/26; 7.6%) developed. Common metastatic sites are regional lymph nodes, the liver, peritoneum, and greater omentum.
Regarding prognosis, Nishihara reported SPT must be classified as lesions of uncertain malignant potential due to varying histological features[13]. As shown in this study, enucleation and partial pancreatectomy is as good as distal pancreatectomy, although surgical oncologic principles are not followed in the former. Based on the clinical follow up, there does not appear to be any difference in survival based on resection techniques. So we suggest that SPT is indolent and surgery for SPT should induce minimal functional deficit. Furthermore, the prognosis is good with 96.4% 5-year survival rate for 26 SPTs. After complete removal of SPT, more than 95% of the patients with SPT are cured. Local spread or dissemination to the peritoneal cavity has been reported due to abdominal trauma and rupture of the tumor. Even in patients who had local spread, recurrent or metastases, long disease-free survival has been recorded after initial diagnosis and resection. Only a few patients have died of metastasizing SPTs.
In conclusion, we present the clinical features and outcomes of 26 SPT patients with emphasis on MRI findings. SPT had a favorable survival irrespective of surgical procedures, malignancy, and MRI pictures. MRI could reliably correlate with its clinicopathological features with specific imaging features.
S- Editor Wang J L- Editor Li M E- Editor Zhou T
| 1. | Klöppel G, Lüttges J, Klimstra DS, Hruban R, Kern S, Adler G. Solid-pseudopapillary neoplasm. World Health Organization classification of tumours: pathology and genetics of tumours of the digestive system. Lyon: IARC Press 2000; 246-248. |
| 2. | Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 3. | Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Raffel A, Cupisti K, Krausch M, Braunstein S, Tröbs B, Goretzki PE, Willnow U. Therapeutic strategy of papillary cystic and solid neoplasm (PCSN): a rare non-endocrine tumor of the pancreas in children. Surg Oncol. 2004;13:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Washington K. Solid-pseudopapillary tumor of the pancreas: challenges presented by an unusual pancreatic neoplasm. Ann Surg Oncol. 2002;9:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Lam KY, Lo CY, Fan ST. Pancreatic solid-cystic-papillary tumor: clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg. 1999;23:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Coleman KM, Doherty MC, Bigler SA. Solid-pseudopapillary tumor of the pancreas. Radiographics. 2003;23:1644-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Lee YR, Kim Y, Koh BH, Cho OK, Rhim H, Park DW, Park HK. Solid and papillary epithelial neoplasm of the pancreas with peritoneal metastasis and its recurrence: a case report. Abdom Imaging. 2003;28:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Jeng LB, Chen MF, Tang RP. Solid and papillary neoplasm of the pancreas. Emphasis on surgical treatment. Arch Surg. 1993;128:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Lee DH, Yi BH, Lim JW, Ko YT. Sonographic findings of solid and papillary epithelial neoplasm of the pancreas. J Ultrasound Med. 2001;20:1229-1232. [PubMed] |
| 11. | Buchino JJ. Fine-needle aspiration of solid and papillary cystic tumor of the pancreas. Pediatr Pathol Lab Med. 1996;16:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Nadler EP, Novikov A, Landzberg BR, Pochapin MB, Centeno B, Fahey TJ, Spigland N. The use of endoscopic ultrasound in the diagnosis of solid pseudopapillary tumors of the pancreas in children. J Pediatr Surg. 2002;37:1370-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Nishihara K, Nagoshi M, Tsuneyoshi M, Yamaguchi K, Hayashi I. Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Cancer. 1993;71:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |