Published online Mar 28, 2007. doi: 10.3748/wjg.v13.i12.1794
Revised: January 26, 2006
Accepted: March 16, 2007
Published online: March 28, 2007
AIM: To investigate whether serum vascular endothelial growth factor-C (SVEGF-C), VEGF-C, and lymphatic vessel density (LVD) in tumor tissues are related to lymph node metastasis (LNM) and prognosis in gastric cancer.
METHODS: SVEGF-C levels of 80 gastric cancer patients and 20 healthy donors were examined using ELISA. VEGF-C expression and LVD were examined using immunohistochemical staining. Kaplan-Meier survival analysis was performed to determine their influence on the prognosis of the patients.
RESULTS: The SVEGF-C level in gastric cancer patients (595.9 ± 201.0 ng/L) was significantly higher (P = 0.000) than controls (360.0 ± 97.4 ng/L). Both SVEGF-C and LVD were significantly higher in poorly differentiated adenocarcinomas, T3 and T4, LNM, distant metastasis, and pTNM groups III and IV (P = 0.000). The sensitivity and specificity of SVEGF-C for predicting LNM were 82.8% and 81.8%, respectively (cut-off = 542.5 ng/L). The positive expression rate of VEGF-C was significantly higher in cancerous than in normal tissues (65% vs 20%; P = 0.001). VEGF-C expression up-regulation was significantly related to differentiation, depth of invasion, LNM, distant metastasis, and pTNM stage (P = 0.000). LVD was 10.7 ± 3.1/200 HP in the experimental group vs 4.9 ± 1.3/200 HP in controls (P = 0.000); LVD in cancerous tissues with and without LNM was 12.0 ± 2.7/200 HP vs 7.6 ± 0.5/200 HP, respectively (P = 0.000). SVEGF-C and LVD were significantly higher in VEGF-C positive than in negative patients (P = 0.000); SVEGF-C level was related to LVD (P = 0.000). Kaplan-Meier survival analysis factors predicating poor prognosis were: SVEGF-C level (P = 0.001), VEGF-C expression and LVD (both P = 0.000).
CONCLUSION: SVEGF-C level, VEGF-C and LVD are related to LNM and poor prognosis of patients with gastric cancer. SVEGF-C may be a biomarker for LNM in gastric cancer.
- Citation: Wang TB, Deng MH, Qiu WS, Dong WG. Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol 2007; 13(12): 1794-1798
- URL: https://www.wjgnet.com/1007-9327/full/v13/i12/1794.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i12.1794
Vascular endothelial growth factor (VEGF) is involved in the neoangiogenesis of solid tumors[1,2]. VEGF-C is a member of the VEGF family that can induce lymphangiogenesis, a critical factor in the progression of many malignant tumors[3-6]. Although the mechanism of lymphangiogenesis and the role of the new lymphatic vessels remain unclear, experimental studies indicate that lymphangiogenic factor VEGF-C may stimulate lymphangiogenesis[7-9]. Several reports have also demonstrated a relationship between VEGF-C expression and lymph node metastasis (LNM) in many cancers, including gastric cancer, oral squamous cell cancer, squamous cell cancer of the esophagus, and non-small cell lung cancer[10-13]. Kitadai et al[14] reported that podoplanin is a specific antibody for lymphatic vessel density (LVD), suggesting that LVD correlates with the clinicopathological factors of patients with gastric cancer. Our research has also shown that podoplanin can be used to identify LVD and lymphangiogenesis is related to LNM of colorectal cancer[15].
In a study of patients with lung cancer, Tamura et al reported that circulating SVEGF-C levels may provide additional information to distinguish between the absence and presence of LNM[16]. Other studies have shown that the SVEGF-C level was higher in patients with cervical cancer than in healthy controls, however, the higher levels did not correlate with LNM[17,18]. In patients with non-small cell lung cancer, both the assay of SVEGF-C, matrix metalloproteinase-9, VEGF and CT examination has been shown to be a clinically useful indictor to predict the presence of LNM[19,20].
To date, few studies have demonstrated whether SVEGF-C level, VEGF-C expression and LVD in tumor tissues indicate the presence of LNM and can predict survival of patients with gastric cancer. In this study, we investigated the circulating level of SVEGF-C, VEGF-C expression and LVD to determine whether they indicate prognosis in patients with gastric cancer.
From October 2002 to October 2003, 80 patients (54 men and 26 women) with a mean age of 57.1 years (± 16.3 SD; range 19-87 years) underwent radical gastrectomy for gastric cancer in the third and first affiliated hospitals of Sun Yat-Sen University. The paraffin embedded specimens of these 80 gastric cancers and 20 normal gastric tissue specimens were collected. Of the 80 gastric cancer specimens, 9 were fungating, 61 ulcerative, and 10 infiltrating; 30 were well differentiated adenocarcinomas, 6 moderately differentiated, and 44 poorly differentiated. The invasive depths were: 7 , 29T2, 36T3 and 8 T4. LNM was found in 58 cases, and 11 patients had distant metastasis. The patients were staged according to surgical and pathological findings using the UICC TNM classification: 19 in stageI, 13 in stage II, 36 in stage III, and 12 in stage IV. Seventy-eight patients followed up for at least 8 mo (mean 37.1 mo; range 8-49 mo) either by telephone or mail. Informed consent was obtained from all of the patients. Twenty patients with benign gastric disease cases served as a control group.
Peripheral venous blood samples were collected according to standard hospital procedure. Serum tubes were centrifuged at 3500 r/min for 10 min at 4°C and then were separated and stored at -70°C. The SVEGF-C level was determined using a quantitative sandwich enzyme immunoassay technique (Bender MedSystems, MedSystems Diagnosis, Vienna, Austria) according to the manufacturer's guidelines. The system uses a solid phase monoclonal antibody and an enzyme-linked polyclonal antibody against recombinant human VEGF-C. For each analysis 100 μL of serum was used. All analyses and calibrations were performed in duplicate. The recombinant human VEGF-C standards were measured to form a standard curve. Results were expressed in ng/L. The coefficient of variation of interassay determinations reported by the manufacturer was < 5.0%. The limits of sensitivity of the VEGF-C assay were 46.9 ng/L.
Specimens were fixed in a 10% formaldehyde solution and embedded in paraffin. Sections 4 μm thick were cut and mounted on glass slides. Immunohistochemical staining was performed using the avidin-biotin method. The podoplanin antibody, rat anti-VEGF-C monoclonal antibody, rat SP and DAB kits were provided by Beijing Zhongshan Biotechnology Co., LTD.
The formalin-fixed, paraffin-embedded 4 μm tissue sections were deparaffinized with xylene, dehydrated in ethanol, and incubated with 3% hydrogen peroxidase for 5 min. After being washed with phosphate buffered saline (PBS), tissue sections were incubated in 10% normal bovine serum for 20 min, followed by an overnight incubation with podoplanin (1:100) or anti-VEGF-C (1:150) antibody. Biotinylated goat antimouse immunoglobulins were used as secondary antibodies. Peroxidase-conjugated avidin was used in a dilution of 1:500. Finally, 0.2 g/L diaminobenzidine and 10 mL/L hydrogen peroxide in PBS were used as the substrate. Specimens positive for podoplanin and VEGF-C were used as positive controls and with the first antibody substituted by PBS as negative controls.
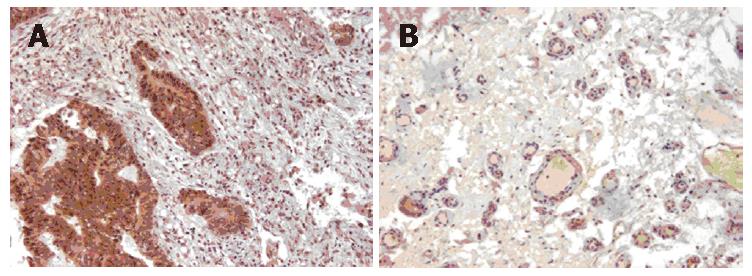
Brown granules in the cytoplasm of a tumor cell were considered indicative of a positive cell. For the evaluation of VEGF-C expression, immunostaining was classified into two groups, corresponding to the percentage of immunoreactive cells. The cut-off point to distinguish negative from positive expression was 30% (Figure 1A). LVD was determined based on brown staining of endothelial cells with podoplanin under a 200-fold light microscope field. Any single brown stained cell or cluster of endothelial cells that was clearly separated from the adjacent microvessel, was considered a lymphatic vessel (Figure 1B). A mean lymphatic vessel of 4 different hot regions was regarded as the LVD of the specimen. Two pathologists who were blinded to data did the observation. The average of microvessels with FVIIIRag staining in 10 hot regions was calculated as MVD.
The Chi-square test or Fisher exact test was used to compare the categorical variables. The Student t test was used to analyze continuous variables. Pearson’s rank correlation test was used to determine the relationship between SVEGF-C level and LVD. The cut-off value of SVEGF-C level to determine LNM was established according to ROC curve. Kaplan-Meier survival analysis was used to estimate survival time and the long-rank test was used to compare the differences in it. Statistical analyses were performed using the SPSS software (SPSS 11.5, SPSS Inc., Chicago, IL, USA). Results were considered statistically significant at P < 0.05.
The SVEGF-C level was significantly (P = 0.000) higher in patients with gastric cancer (595.9 ± 201.0 ng/L) than in healthy donors (360.0 ± 97.4 ng/L). With a cut-off value for SVEGF-C of 367.5 ng/L, the sensitivity and specificity for diagnosis of gastric cancer patients was 85% and 80%, respectively (P = 0.000). VEGF-C positive expression was significantly (P = 0.001) higher in gastric cancer tissue (50/80) than in normal gastric tissue (4/20), There was significantly (P = 0.000) more LVD in the experimental group (10.7 ± 3.1/200 HP) than in control subjects (4.9 ± 1.3/200 HP).
SVEGF-C level, VEGF-C expression, and LVD were associated with the clinicopathologic features of gastric cancer (Table 1). The level of SVEGF-C reached the highest sensitivity (82.8%) and specificity (81.8%) in diagnosis of LNM when a cut-off value of 542.5 ng/L was used (P = 0.000).
| Pathological characteristics | n | SVEGF-C(ng/L) | VEGF-C | LVD(/200HP) | |
| + | - | ||||
| Differentiation degree | |||||
| G1+G2 | 36 | 427.9 ± 130.0 | 9 | 27 | 7.9 ± 0.9 |
| G3 | 44 | 733.4 ± 130.0c | 41 | 3a | 13.1 ± 2.2c |
| Depth of invasion | |||||
| T1+T2 | 36 | 536.1 ± 227.4 | 14 | 32 | 9.8 ± 2.8 |
| T3+T4 | 44 | 644.9 ± 161.5a | 36 | 8c | 11.5 ± 3.2b |
| LNM | |||||
| Yes | 58 | 650.9 ± 198.6 | 46 | 12 | 12.0 ± 2.7 |
| No | 22 | 451.0 ± 115.5c | 4 | 18c | 7.6 ± 0.5c |
| Distant metastasis | |||||
| Yes | 11 | 834.3 ± 80.0 | 10 | 1 | 14.0 ± 1.2 |
| No | 69 | 558.0 ± 187.0c | 40 | 29c | 10.2 ± 3.0c |
| pTNM stage | |||||
| I+ II | 32 | 412.5 ± 130.0 | 7 | 25 | 7.7 ± 0.9 |
| III+ IV | 48 | 718.2 ± 134.4c | 43 | 5c | 12.7 ± 2.4c |
The SVEGF-C level was significantly (P = 0.000) higher in VEGF-C positive patients (675.4 ± 153.9 ng/L) than in negative patients (463.5 ± 200.4 ng/L). There was a positive correlation between SVEGF-C and LVD (r = 0.728, P = 0.000). LVD in VEGF-C positive and negative groups was 12.2 ± 2.8/200 HP and 8.3 ± 2.0/200 HP, respectively (P = 0.000).
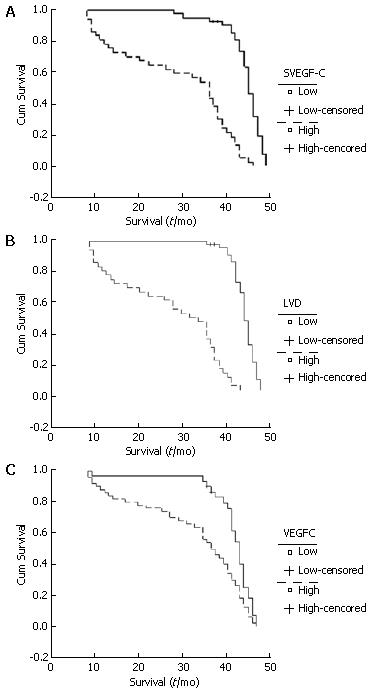
In this study, the 3-year survival rate was 56.3%. The mean survival of patients with high (> 595.9 ng/L) SVEGF-C and low (< 595.9 ng/L) SVEGF-C was 29.1 ± 13.3 mo and 44.0 ± 4.6 mo, respectively (P = 0.001; Figure 2A). Patients in the high (> 10.7/200 HP) LVD group had a mean survival of 27.4 ± 12.6 mo, while the mean survival time in low (< 10.7/200 HP) LVD group was 44.7 ± 3.1 mo (P = 0.000; Figure 2B). The mean survival time was shorter in the VEGF-C positive expression group than in the negative expression group (33.8 ± 13.3 mo vs 42.6 ± 7.4 mo, P = 0.000; Figure 2C).
VEGF-C, a member of VEGF family, is known to bind to vascular endothelial growth factor receptor-3 on lymphatic endothelial cells and promote lymphangiogenesis[1-3]. Several studies have found that in many cancers, the positive expression of VEGF-C is higher in tumor tissue than in normal tissue and higher in positive LNM groups than in negative ones[9-12]. Arinaga et al[13] have shown that patients with non-small cell lung cancer and positive staining for VEGF-C had unfavorable survival rates. Our early research showed that VEGF-C expression was stronger in colorectal cancer tissue than in normal colorectal tissue and that there was a relationship between VEGF-C expression and LNM[15]. The higher rate of VEGF expression in cancerous tissue in the present study clearly demonstrates that VEGF-C over-expression exists in gastric cancer. This study also shows that VEGF-C expression is related to the clinicopathologic features of gastric cancer. These results strongly indicate that VEGF-C may promote lymph metastasis and progression of gastric cancer. VEGF-C produced from cancer cells may increase the expression of vascular endothelial growth factor receptor-3 on the lymphatic endothelial cells and promote the formation of new lymphatic vessels. The present study indicates that positive expression of VEGF-C in tumor tissue is an unfavorable factor for the survival of gastric cancer patients. This is consistent with other studies indicating that VEGF-C expression is an independent factor in the prognosis of patients with gastric cancer[21-23]. Given these data, it is reasonable to assume that a high expression of VEGF-C may be a biomarker for the development of gastric cancer and may predict unfavorable survival rates in patients with this malignancy.
In the present study, the SVEGF-C level is higher in patients with gastric cancer than in healthy controls, which is consistent with results from studies in patients with other cancers, including lung, esophageal, and colorectal cancer[16,24,25]. Tamura et al[16] found that the SVEGF-C level was higher in patients with non-small cell lung cancer with LNM than in the those without. Similarly, Krzystek-Korpacka et al[21] observed that SVEGF-C was a biomarker of LNM in esophageal cancer and Ichikura et al found that patients with colorectal cancer with LNM had a higher SVEGF-C level than those without LNM[22]. Another study, however, conducted in patients with advanced cervical cancer did not show a correlation between pretherapeutic levels of VEGF-C and LNM[18]. It has been suggested that circulating VEGF-C level might provide additional information to distinguish between the absence and presence of LNM in patients with cancer. We found that SVEGF-C was related to VEGF-C expression in patients with gastric cancer. Mathur et al[17] reported similar results in patients with cervical cancer. To the best of our knowledge, however, this is the first report identifying SVEGF-C level as a marker for LNM and an unfavorable factor for the prognosis of patients with gastric cancer.
Neogenesis of lymphatic vessel and lymphatic invasion is frequently found in the stroma of cancers, while the presence and role of intratumoral lymphatic vessels have been controversial. In an earlier study, we found that LVD was higher in cancerous colorectal tissue than in normal specimens and a significant correlation with the pathological features of the disease[15]. The present study demonstrates similar results in gastric cancer, and LVD was higher in patients with LNM than without and higher in the VEGF-C positive group than in the VEGF-C negative group. We also found a significant correlation between SVEGF-V level and LVD, and that LVD predicated a poorer prognosis of patients with gastric cancer. Previous research has already demonstrated that LVD is correlated with LNM in human gastric carcinoma[14]. Skobe et al[26] demonstrated the occurrence of intratumoral lymphangiogenesis within human breast cancers and Munoz-Guerra et al[27] documented a strong association between LVD and loco-regional recurrence in patients with oral cancer. Kitadai et al[14] reported that LVD in patients with gastric cancer was significantly higher in those with LNM, and the high LVD was highly correlated with VEGF-C expression, while Massi et al[28] reported that LVD was significantly higher in melanomas with LNM metastasis than in non-metastatic melanomas. However, there has been controversy about the number and role of LVD in malignant diseases. Brundler et al[29] showed that LVD was significantly reduced in adenocarcinoma, being half that in the normal stomach. In prostate adenocarcinoma, LVD was significantly lower than the peritumoral and normal prostate, and was not significantly different between patients with and without LNM[30]. Massi et al[28] found that peritumorous LVD was an independent variable affecting overall survival.
In summary, SVEGF-C level, VEGF-C expression and LVD in gastric cancer may be prognostic parameters to identify patients with poor outcome. Preoperative SVEGF-C level might be a useful biomarker for judging the presence of LNM in patients with gastric cancer.
There are few studies which demonstrated simultaneously whether serum vascular endothelial growth factor-C (SVEGF-C) level, VEGF-C expression, and lymphatic vessel density (LVD) indicate LNM and whether this influences the survival of patients with gastric cancer. The authors investigated the lymphangiogenesis factor to determine if it was related to the progression and prognosis of patients with gastric cancer.
To date, the mechanism of lymphangiogenesis and role of new lymphatic vessel have been unclear. Many studies indicated that lymphangiogenic factor VEGF-C could stimulate lymphangiogenesis. Some reports indicated that LVD was related to the clinicopathological features in many cancers. It has also been reported, although controversially, that circulating SVEGF-C level may provide additional information for distinguishing between the absence and presence of LNM in patients with cancer.
In the present study, the authors examined SVEGF-C, VEGF-C expression and LVD in gastric cancer using ELISA and immunohistochemical staining. It was found that not only SVEGF-C but also VEGF-C expression and LVD in tumor tissues were related to LNM and survival of patients with gastric cancer. These three factors in gastric cancer have been investigated simultaneously for the first time.
The results of the present study show that SVEGF-C level, VEGF-C expression, and LVD in tumor tissue are related to both progression and prognosis of patients with gastric cancer. These findings may help understand, to some degree, the role of lymphangiogenesis in this malignant disease. All the three parameters could provide information on the development and prognosis of patients with gastric cancer. And preoperatively high SVEGF-C level might be a biomarker for the occurrence of LNM in gastric cancer.
Lymphangiogenesis: the mechanisms of lymphatic vessel formation. The lymphatic vasculature is important for transporting macromolecules from the interstitial space. The LNM of malignant tumors is perhaps based on the dissemination of tumor cells via the lymphatic vessels. Studies showed that Prox-1, VEGF-C and VEGF-A are required for the budding and sprouting of lymphatic vessels. Currently, the exact mechanism of lymphatic formation is unclear.
Wang and coworkers looked at serum VEGF-C and lymphatic vessel density in gastric cancer, and found a positive correlation both with lymph node metastasis and poor prognosis of the patients with gastric cancer. The paper is of some interest and importance.
S- Editor Wang J L- Editor Ma JY E- Editor Chin GJ
| 1. | Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591-595. [PubMed] |
| 2. | Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994-998. [PubMed] |
| 3. | Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290-298. [PubMed] |
| 4. | Enholm B, Jussila L, Karkkainen M, Alitalo K. Vascular endothelial growth factor-C: a growth factor for lymphatic and blood vascular endothelial cells. Trends Cardiovasc Med. 1998;8:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Mimura T, Amano S, Usui T, Kaji Y, Oshika T, Ishii Y. Expression of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in corneal lymphangiogenesis. Exp Eye Res. 2001;72:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Ueda M, Terai Y, Kumagai K, Ueki K, Yamaguchi H, Akise D, Ueki M. Vascular endothelial growth factor C gene expression is closely related to invasion phenotype in gynecological tumor cells. Gynecol Oncol. 2001;82:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Yonemura Y, Fushida S, Bando E, Kinoshita K, Miwa K, Endo Y, Sugiyama K, Partanen T, Yamamoto H, Sasaki T. Lymphangiogenesis and the vascular endothelial growth factor receptor (VEGFR)-3 in gastric cancer. Eur J Cancer. 2001;37:918-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, Chayama K. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer. 2002;38:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Liu XE, Sun XD, Wu JM. Expression and significance of VEGF-C and FLT-4 in gastric cancer. World J Gastroenterol. 2004;10:352-355. [PubMed] |
| 10. | Yan C, Zhu ZG, Yu YY, Ji J, Zhang Y, Ji YB, Yan M, Chen J, Liu BY, Yin HR. Expression of vascular endothelial growth factor C and chemokine receptor CCR7 in gastric carcinoma and their values in predicting lymph node metastasis. World J Gastroenterol. 2004;10:783-790. [PubMed] |
| 11. | Kishimoto K, Sasaki A, Yoshihama Y, Mese H, Tsukamoto G, Matsumura T. Expression of vascular endothelial growth factor-C predicts regional lymph node metastasis in early oral squamous cell carcinoma. Oral Oncol. 2003;39:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Noguchi T, Takeno S, Shibata T, Uchida Y, Yokoyama S, Müller W. VEGF-C expression correlates with histological differentiation and metastasis in squamous cell carcinoma of the esophagus. Oncol Rep. 2002;9:995-999. [PubMed] |
| 13. | Arinaga M, Noguchi T, Takeno S, Chujo M, Miura T, Uchida Y. Clinical significance of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in patients with nonsmall cell lung carcinoma. Cancer. 2003;97:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, Tanaka S, Matsumura S, Yasui W, Chayama K. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer. 2005;115:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Wang TB, Huang YH, Lan P, Song XM, Wang JP. Correlation of lymphangiogenesis to progression of colorectal cancer. Ai Zheng. 2005;24:1276-1279. [PubMed] |
| 16. | Tamura M, Ohta Y. Serum vascular endothelial growth factor-C level in patients with primary nonsmall cell lung carcinoma: a possible diagnostic tool for lymph node metastasis. Cancer. 2003;98:1217-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Mathur SP, Mathur RS, Gray EA, Lane D, Underwood PG, Kohler M, Creasman WT. Serum vascular endothelial growth factor C (VEGF-C) as a specific biomarker for advanced cervical cancer: Relationship to insulin-like growth factor II (IGF-II), IGF binding protein 3 (IGF-BP3) and VEGF-A [corrected]. Gynecol Oncol. 2005;98:467-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Mitsuhashi A, Suzuka K, Yamazawa K, Matsui H, Seki K, Sekiya S. Serum vascular endothelial growth factor (VEGF) and VEGF-C levels as tumor markers in patients with cervical carcinoma. Cancer. 2005;103:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Tamura M, Oda M, Matsumoto I, Tsunezuka Y, Kawakami K, Ohta Y, Watanabe G. The combination assay with circulating vascular endothelial growth factor (VEGF)-C, matrix metalloproteinase-9, and VEGF for diagnosing lymph node metastasis in patients with non-small cell lung cancer. Ann Surg Oncol. 2004;11:928-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Tamura M, Oda M, Tsunezuka Y, Matsumoto I, Kawakami K, Ohta Y, Watanabe G. Chest CT and serum vascular endothelial growth factor-C level to diagnose lymph node metastasis in patients with primary non-small cell lung cancer. Chest. 2004;126:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K. Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res. 1999;5:1823-1829. [PubMed] |
| 22. | Ichikura T, Tomimatsu S, Ohkura E, Mochizuki H. Prognostic significance of the expression of vascular endothelial growth factor (VEGF) and VEGF-C in gastric carcinoma. J Surg Oncol. 2001;78:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Takahashi A, Kono K, Itakura J, Amemiya H, Feng Tang R, Iizuka H, Fujii H, Matsumoto Y. Correlation of vascular endothelial growth factor-C expression with tumor-infiltrating dendritic cells in gastric cancer. Oncology. 2002;62:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Banas T. Up-regulation of VEGF-C secreted by cancer cells and not VEGF-A correlates with clinical evaluation of lymph node metastasis in esophageal squamous cell carcinoma (ESCC). Cancer Lett. 2007;249:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Xu T, Chen D. Serum vascular endothelial growth factor-C and vascular endothelial growth factor level in patients with colorectal carcinoma and clinical significance. J Huazhong Univ Sci Technolog Med Sci. 2006;26:329-331, 355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1298] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 27. | Muñoz-Guerra MF, Marazuela EG, Martín-Villar E, Quintanilla M, Gamallo C. Prognostic significance of intratumoral lymphangiogenesis in squamous cell carcinoma of the oral cavity. Cancer. 2004;100:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Massi D, Puig S, Franchi A, Malvehy J, Vidal-Sicart S, González-Cao M, Baroni G, Ketabchi S, Palou J, Santucci M. Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: a case-control study. J Clin Pathol. 2006;59:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Brundler MA, Harrison JA, de Saussure B, de Perrot M, Pepper MS. Lymphatic vessel density in the neoplastic progression of Barrett's oesophagus to adenocarcinoma. J Clin Pathol. 2006;59:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Roma AA, Magi-Galluzzi C, Kral MA, Jin TT, Klein EA, Zhou M. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod Pathol. 2006;19:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |