Published online Mar 28, 2007. doi: 10.3748/wjg.v13.i12.1788
Revised: December 3, 2006
Accepted: February 26, 2007
Published online: March 28, 2007
AIM: To investigate the synergistic effect of oxymatrine (OM) and angiogenesis inhibitor NM-3 on modulating apoptosis in human gastric cancer cell lines SGC-7901, MKN-45, MKN-74.
METHODS: Human gastric cancer lines SGC-7901, MKN-45, MKN-74 were treated with OM in the absence and presence of NM-3. The inhibitory rates were detected by MTT assay. Synergistic effect of OM and NM-3 on the growth of survivin, bcl-2, bax and p53 in SGC-7901 cells were examined by semiquantitative RT-PCR and Western blotting, and their growth inhibitory effects were also observed on SGC-7901 tumor xenograft in nude mice.
RESULTS: OM combined with NM-3 exhibited a synergistic inhibitory effect on the growth of SGC-7901, MKN-45 and MKN-74 cells in a time-dependent manner. Twenty-four hours after treatment with OM, NM-3 alone and their combination, mRNA expression of survivin and bcl-2 in SGC-7901 cells decreased, p53 mRNA expression increased. OM (4 g/L) combined with NM-3 significantly increased the expression of p53 mRNA and decreased the expression of survivin and bcl-2 compared with either agent alone (193% ± 34% vs 129% ± 12%; 44% ± 18% vs 92% ± 18%; 36 ± 17% vs 93% ± 23%, P < 0.05). Western blotting showed that the synergistic effect of OM and NM-3 on protein translation of survivin, bcl-2 and p53 was in accordance with their mRNAs. Furthermore, OM/NM-3 combination obviously exhibited antitumor growth effect in xenografted human gastric cancer cells SGC-7901 compared with either agent alone.
CONCLUSION: OM combined with NM-3 has synergistic inhibitory effects on human gastric cancer cells in vitro and can suppress the growth of xenografted human gastric cancer cells SGC-7901 in vivo.
- Citation: Song MQ, Zhu JS, Chen JL, Wang L, Da W, Zhu L, Zhang WP. Synergistic effect of oxymatrine and angiogenesis inhibitor NM-3 on modulating apoptosis in human gastric cancer cells. World J Gastroenterol 2007; 13(12): 1788-1793
- URL: https://www.wjgnet.com/1007-9327/full/v13/i12/1788.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i12.1788
NM-3 is a novel angiogenesis inhibitor isolated from a culture filtrate of streptoverticillium eurocidicum[1-3]. Studies indicated that NM-3 is an effective inhibitor of angiogenesis[4]. Subsequent work has shown that NM-3 also exhibits direct cytotoxic effects on endothelial and carcinoma cells[5]. Moreover, NM-3 has been found to potentiate the therapeutic effects of certain chemotherapeutic agents, including 5-fluorouracil, paclitaxel, and cyclophosphamide[6]. Our previous study showed that NM-3 combined with carboplatin can suppress the growth of human gastric cancer SGC-7901 cells xenografted in nude mice[7], suggesting that NM-3 combined with carboplatin could not only increase anticancer effect but also aggravate toxicity. In this study, we selected oxymatrine (OM) to substitute carboplatin for its lower toxic side-effect and its ability to kill human cancer cells[8-10]. The purpose of this study was to investigate whether OM combined with NM-3 has synergistic inhibitory effects on human gastric cancer cells and growth of xenografted human gastric cancer cells SGC-7901.
NM-3 supplied by Professor Robert (New York University) was dissolved in PBS sterilized at a stock concentration of 1 mg/mL. For in vivo experiments, NM-3 (10 mg/kg per d) was administered via i.p. injection. OM was purchased from Shanghai Huamei Corporation (China). MTT purchased from Sigma (USA) was dissolved in 0.01 mol/L PBS. TRIzol reagent was from Gibico BRL Co (Germany). M-MuLV reverse transcription-polymerase chain reaction kit was from TaKaRa (Japan). One-step RNA PCR kit was obtained from AMV TaKaRa (Japan). Survivin and p53 polyclonal antibodies were purchased from Santa Cruz Co (USA). Bcl-2 and bax antibodies were obtained from San Francisco (CA). PCR primers were synthesized in Shanghai Sangon (China).
Gastric cancer cell lines SGC-7901 and MKN-45 and MKN-74 were purchased from Wuhan University and maintained at 37°C in DMEM supplemented with 10% FCS and 2 mmol/L glutamine in a humidified incubator containing 50 mL/L CO2 until they were amplified to a certain concentration 6 × 106/mL.
Thirty-two 6-7 wk old BALB/C-nu/nu mice weighing 18-22 g were obtained from Shanghai Laboratory Animal Center, Chinese Academy of Sciences (SPF, NO.SCXK033).
Cell growth was assessed by MTT assay. A total of 5 × 103 cells were seeded into each well of 96-well culture plates in RPMI 1640 medium for 24 h, treated with scalar concentrations of OM in the absence or presence of 10 mg/L NM-3, and incubated for 24, 48, 72 and 96 h respectively. After incubation, 10 μL of MTT solution (5 g/L) was added to each well, and the plates were then incubated for 3 h at 37°C. The growth medium was then replaced with 150 μL of dimethyl sulfoxide (Wako) per well, and the absorbance at A490 was measured using Titertek Multiscan. IC50 with SAS statistical software.
One μg of total RNA from each sample was resuspended in a 25 μL final volume of reaction buffer, containing 10 × one-step RNA PCR buffer, 25 mmol/L MgCl2, 10mmol/L dNTP mixture, 20 U RNase inhibitor, 2.5U AMV RTase XL, 2.5 U AMV-Optimized Taq, 20 μmol/L forward primer, 20 μmol/L reverse primer, and RNase-free dH2O. Following primers were for survivin (349 bp): 5’-GGA CCA CCG CAT CTC TAC AT-3’, 3’-CC TTT GAC GCT TCT TTC ACG-5; bcl-2 (292 bp): 5’-TGC ACC TGA CGC CCT TCA C-3’, 3’-G ACA CTA AAG AGG ACC GAC AGA-5’; bax (364 bp): 5’-ACC AAG AAG CTG AGC GAG TGT C-3’, 3’-C CGT CTG GCA CTG GTA GAA ACA-5’; p53 (396): 5’-CAG CCA AGT CTG TGA CTT GCA CGT AC-3’, 3’-CT ACT GTC TTT GTG AAA AGC TGT ATC-5’; β-actin(542 bp): 5’-GCTCCGGCATGTGCAA-3’, 3’-TGATGGAGTACTTCTAGGA-5’.
The PCR conditions were at 94°C for 5 min, 1 cycle; at 94°C for 30 s, at 59°C (survivin, bcl-2, p53, β-actin) or at 56°C (bax)for 30 s, at 72°C for 45 s, 35 (survivin, bcl-2, p53, β-actin) or 30 (bax) circles; at 72°C for 7 min, 1 circle. The PCR products were electrophoresed on 1.5% agarose gels containing ethidium bromide and revealed by a gel documentation system (FR-200, Shanghai Fu Ri Bio Co. China). The content of cDNA was semi-quantitatively normalized with housekeeping gene β-actin as the internal control.
Gastric cancer SGC-7901 cells were seeded into 250 mL flasks, grown to confluence and incubated in the presence of NM-3 either alone or in combination with OM (1, 2, 4 g/L) for 24 h. Cells were then harvested and lysed in a modified protein lysis buffer (10 mmol/L Tris-HCl, pH 8.0, 135 mmol/L NaCl, 1 mmol/L EDTA, 10mmol/L CHAPS, 10 mg/L aprotinin, 0.02 mmol/L APMSF), and the protein concentration of the lysates was measured. Total cell protein extracts (15 μg/lane) were separated by SDS-PAGE using ready gels (Bio-Rad Laboratories, Milan, Italy) and electrophoretically transferred onto PVDF membranes. The membranes were blocked with 5% dried non-fat milk in PBS buffer and 0.05% Tween20. The filters were then incubated with primary antibodies against survivin, bcl-2, bax and p53. Each of these antibodies was diluted according to the manufacturer’s instructions. Membranes were then washed with PBS-T buffer (PBS with 0.1% Tween 20) and incubated with the appropriate secondary antibodies. Immunoreactive proteins were visualized with enhanced chemiluminescence (Amersham International, Buckinghamshire, UK).
Suspensions of tumor cells SGC-7901 (5-10 × 106 viable cells/mouse) were implanted into the bilateral flank region of BALB/c-nu/nu mice as previously described[7]. When the tumors grew to 50-100 mm3, the animals were randomly divide into four groups (n = 9). The animals were pair matched so that the median tumor volume for each group was similar: control group: NM-3 (10 mg/kg per day) via i.p. injection thrice per week; treatment groups: OM (1, 2, 4 g/L and NM-3 (10 mg/kg per day) via i.p. injection thrice per week. The tumor volumes were estimated by V = ab2/2, where a and b are tumor length and width, respectively. To evaluate the antitumor effects of OM and NM-3, tumor size and body were measured thrice per week.
The results were expressed as mean ± SD. Statistical analysis was performed using Chi-square test , Student’s t test. P < 0.05 was considered statistically significant.
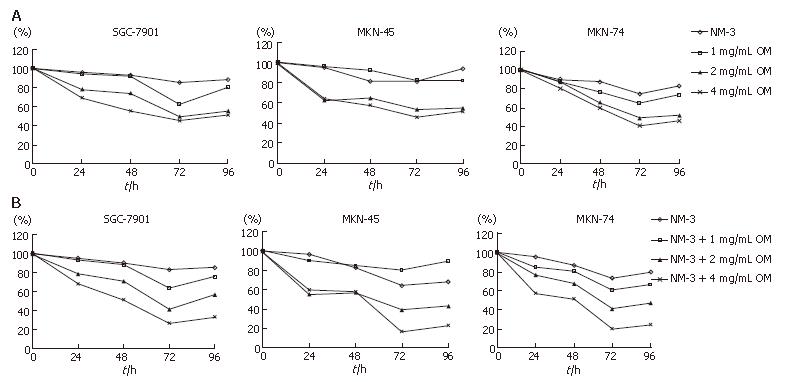
We analyzed the synergistic growth inhibitory effects of OM and NM-3 on the gastric cancer cell lines SGC-7901, MKN-45 and MKN-74. MTT assays were performed to examine the growth inhibitory effect of OM or NM-3 alone (Figure 1A) and OM in combination with NM-3 (Figure 1B). Each cell line was treated with 1, 2 and 4 g/L OM, either alone or in combination with NM-3. The time-course effects were observed 24, 48, 72 and 96 h after treatment. MTT assays were performed to analyze the inhibitory effects of the drugs at each time point, which were compared to the non-treated control cells. Treatment with OM alone and combined OM/NM-3 inhibited the growth of three gastric cancer cell lines in a time- and dose- dependent manner.
The OM IC50 levels were found to be 2.51, 3.02 and 2.32 mg/mL for SGC-7901, MKN-45 and MKN-74, respectively. To determine the potent effects of OM on NM-3 activity, the growth inhibitory effects of NM-3 alone or in combination with OM on three gastric cancer cell lines were evaluated. After treatments the IC50 of NM-3 for each cell line was compared with that of NM-3 in combination with 4 g/L OM (Table 1). These values were significantly reduced by 2.54-, 4.13- and 3.38-fold in each case, suggesting that OM in combination with NM-3 had synergistic effects on growth of gastric cancer cells.
| NM-3 alone | NM-3 plus OM | Fold | |
| SGC-7901 | 321.21 ± 51.21 | 132.84 ± 21.13 | 2.54 |
| MKN-45 | 245.56 ± 31.34 | 66.07 ± 18.21 | 4.13 |
| MKN-74 | 225.92 ± 33.64 | 71.75 ± 20.11 | 3.38 |
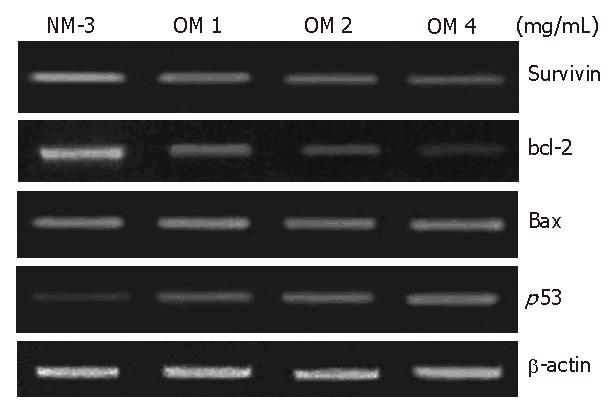
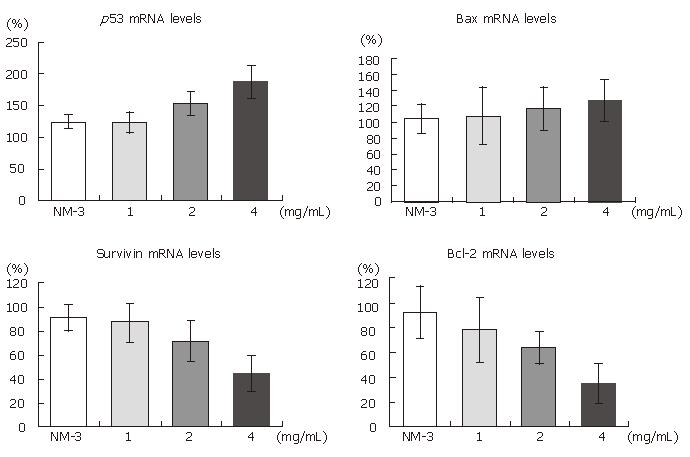
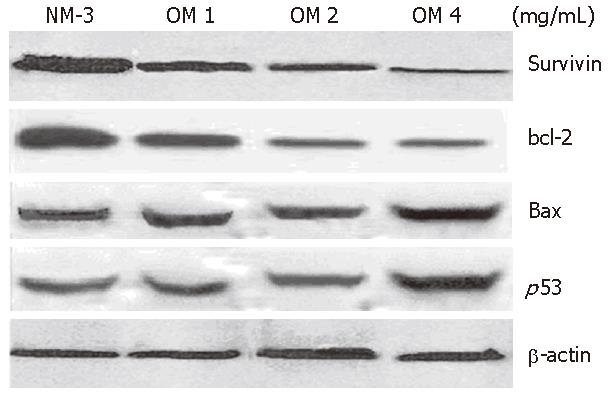
To define the mechanisms of OM and NM-3 in inducing apoptosis, we first studied the changes in mRNA expression of survivin, bcl-2, bax and p53. The mRNA and protein expression levels in SGC-7901 cells were analyzed by RT-PCR and Western blotting. After treatment with NM-3 or with OM (1, 2, 4 g/L) for 24 h, both cDNA and proteins in survivin, bcl-2, bax and p53 in SGC-7901 cells were evaluated.
As shown in Figures 2 and 3, treatment with OM (1 mg/mL, 2 mg/mL, 4 mg/mL) in combination with NM-3 increased the mRNA levels in bax and p53 and decreased the survivin and bcl-2 levels in a dose-dependent manner. Combined 4 mg/mL OM and NM-3 significantly increased p53 mRNA expression (193% ± 34% vs 129% ± 12%, P < 0.05) and significantly decreased survivin and bcl-2 mRNA expression compared to NM-3 alone (44% ± 18% vs 92% ± 18%, 36% ± 17% vs 93% ± 23%, P < 0.05) compared to NM-3 alone.
The expression of p53 protein 24 h after treatment with increased dose of OM was 2.8-fold higher in OM treatment group than in NM-3 treatment group. Survivin expression decreased in a dose-dependent manner. Moreover, the expression of bax protein was slightly increased after treatment with NM-3 alone or in combination with OM. There was a significant difference in p53, survivin and bcl-2 expression between the two groups treated with OM (4 mg/mL) in combination with NM-3 or with NM-3 alone (P < 0.05). These findings strongly suggest ed that synergism between OM and NM-3 induced apoptosis in more gastric cancer cells than NM-3 alone (Figure 4).
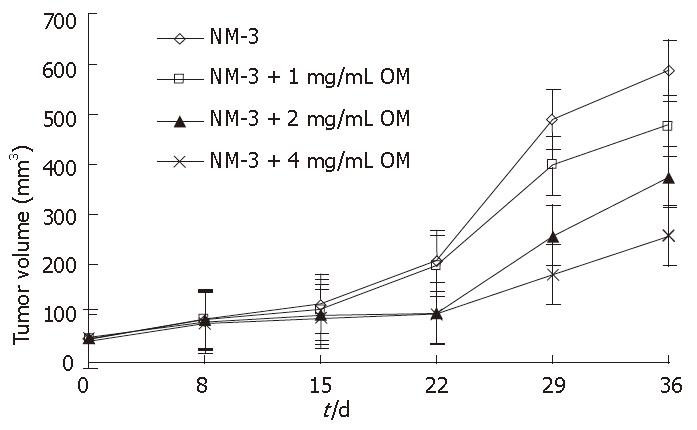
To assess the potential synergistic effects of OM and NM-3 in vivo, human tumor xenograft experiments were performed. We examined the growth inhibitory effects of NM-3 alone and in combination with OM on SGC-7901cells transplanted into nude mice. The resulting growth curves for gastric cancer xenografts during various treatment schedules are shown in Figure 5.
NM-3 alone (10 mg/kg per day) or in combination with OM showed a tumor growth inhibitory rate of 9.5% ± 3.1%, 12.3% ± 3.5%, 40.6% ± 9.7% and 52.8% ± 12.4% on d 15, and 18.8% ± 4.8%, 28.6% ± 8.4%, 53.4% ± 13.5% and 75% ± 18.5% on d 36, respectively. OM in combination with NM-3 had stronger effect than NM-3 alone on tumor growth (P < 0.05). These results further suggested that combined OM and NM-3 had synergistic effects on tumor growth. In addition, none of the treated animals in our experiments showed weight loss due to drug toxicity, demonstrating that combined OM and NM-3 was effective against gastric cancer and could be used as a new therapeutic tool for treatments of tumors. Furthermore, no increase in toxicity was observed after combined treatment.
When NM-3 is combined with other chemotherapeutic agents, it could effectively inhibit the growth of tumor[6]. In our previous studies we used NM-3 (10-40 mg/kg) in combination with carboplatin to treat nude mice xenografted with SGC-7901 cell lines, and found that some mice became emaciated for diarrhea and anorexia in early stage and some died[7]. The death rate was higher than 16% in groups treated with NM-3 (greater than 20 mg/kg). However, all the mice treated NM-3 (10 mg/kg) showed less severe reaction. We substituted OM for carboplatin because OM can kill human cancer cells with less toxic side-effects. It was recently reported that OM induces apoptosis of human hepatoma SMMC-7721 cells and human colorectal tumor SW1116 cells[11,8].
In this study, we analyzed the effects of OM and OM in combination with NM-3 on proliferation of human gastric cancer lines in vitro. OM alone and in combination with NM-3 inhibited the growth of each gastric cancer cell line in a time- and -dependent manner. Moreover, their striking synergistic effects were observed in three gastric cancer cells with NM-3 IC50 values being obviously decreased when combined with OM. Furthermore, in nude mice with SGC-7901 xenografts, treatment with OM and NM-3 exhibited significant synergistic antitumor effects. Our present studies showed that when OM was used in combination with NM-3, a significant reduction in xenograft volumes was observed without an apparent increase in toxicity, demonstrating that combined OM and NM-3 is more effective against gastric cancer.
To further discuss the exact mechanism of apoptosis induced by OM and NM-3, we detected the changes in survivin, bcl-2, bax and p53 genes by semiquantitive PCR. The result showed that OM in combination with NM-3 could down-regulate the expression of survivin, bcl-2 and up-regulate the expression of p53 mRNA. We also validated the similar results from Western blotting, showing that the inhibitory effect of OM in combination with NM-3 is associated with their ability to induce apoptosis by regulating the transcription and translation of survivin, bcl-2 and p53 genes.
Cell apoptosis is induced and controlled by many complicated factors, such as expression changes of correlative apoptosis genes and blockage of cell cycle. Survivin belongs to the inhibitory apoptosis protein family and is a good marker of most cancer cells[12,13]. Various strategies to target survivin in cancer treatment are currently under investigation with promising results in vivo and in vitro[14-22]. Bcl-2 is a central apoptotic suppression gene and overexpresses in most immortal and cancer cells[23]. Most therapeutic agents act by blocking the function of anti-death bcl-2 proteins or by enhancing the activities of pro-death proteins such as bax[24]. Once the brake provided by anti-death bcl-2 members is removed, the pro-death protein bax oligomerizes at the mitochondrial membrane leading to release of apoptogenic factors into the cytosol, an event initiating a deadly proteolytic cascade[25].
P53 is a multifunctional protein with multiple modifications and biochemical properties. p53 has the ability to bind to specific DNA sequences and functions as a potent transcription factor, that trans-activates and trans-represses specific genes involved in controlling cell cycle and apoptosis[26]. Activation of p53 is associated with induction of p21 expression, an event regulated in part by p53-mediated trans-activation. When p21 cells down-regulate Cdk2 and arrest in G1-S-phase[27,28], p53 also acts as a tumor suppression protein opposite to bcl-2. Recent studies indicate that conventional anticancer drugs can perform their functions modulating bcl-2 protein expression, whereas this modulation seems ultimately to lead to the presence of p53[29,30].
In this study, we focused on the synergistic effects of OM and NM-3 on human gastric tumor cells and evaluated their apoptosis regulation in vitro and in vivo. The data show that OM in combination with NM-3 inhibits growth and proliferation of tumor cells, and induces apoptosis of human gastric cancer cells, which is associated with down-regulation of surviving and bcl-2 expression and up-regulation of p53 expression in SGC-7901 cells. In conclusion, our present study strongly indicates that combined OM and NM-3 is synergistically effective against gastric carcinomas.
S- Editor Liu Y L- Editor Wang XL E- Editor Zhou T
| 1. | Bauta WE, Lovett DP, Cantrell WR, Burke BD. Formal synthesis of angiogenesis inhibitor NM-3. J Org Chem. 2003;68:5967-5973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Nakashima T, Hirano S, Agata N, Kumagai H, Isshiki K, Yoshioka T, Ishizuka M, Maeda K, Takeuchi T. Inhibition of angiogenesis by a new isocoumarin, NM-3. J Antibiot (Tokyo). 1999;52:426-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Kumagai H, Masuda T, Ohsono M, Hattori S, Naganawa H, Sawa T, Hamada M, Ishizuka M, Takeuchi T. Cytogenin, a novel antitumor substance. J Antibiot (Tokyo). 1990;43:1505-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Salloum RM, Jaskowiak NT, Mauceri HJ, Seetharam S, Beckett MA, Koons AM, Hari DM, Gupta VK, Reimer C, Kalluri R. NM-3, an isocoumarin, increases the antitumor effects of radiotherapy without toxicity. Cancer Res. 2000;60:6958-6963. [PubMed] |
| 5. | Yin L, Ohno T, Weichselbaum R, Kharbanda S, Kufe D. The novel isocoumarin 2-(8-hydroxy-6-methoxy-1-oxo-1H-2-benzopyran-3-yl) propionic acid (NM-3) induces lethality of human carcinoma cells by generation of reactive oxygen species. Mol Cancer Ther. 2001;1:43-48. [PubMed] |
| 6. | Reimer CL, Agata N, Tammam JG, Bamberg M, Dickerson WM, Kamphaus GD, Rook SL, Milhollen M, Fram R, Kalluri R. Antineoplastic effects of chemotherapeutic agents are potentiated by NM-3, an inhibitor of angiogenesis. Cancer Res. 2002;62:789-795. [PubMed] |
| 7. | Zhu JS, Shen B, Chen JL, Chen GQ, Yu XH, Yu HF, Zhu ZM. Molecule action mechanisms of NM-3 on human gastric cancer SGC-7901 cells in vivo or in vitro. World J Gastroenterol. 2003;9:2366-2369. [PubMed] |
| 8. | Zou J, Ran ZH, Xu Q, Xiao SD. Experimental study of the killing effects of oxymatrine on human colon cancer cell line SW1116. Chin J Dig Dis. 2005;6:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Song G, Luo Q, Qin J, Wang L, Shi Y, Sun C. Effects of oxymatrine on proliferation and apoptosis in human hepatoma cells. Colloids Surf B Biointerfaces. 2006;48:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu XS, Xu XR, Liu BZ, He YJ. Effects of Matrine on proliferation and differentiation in K-562 cells. Leuk Res. 2001;25:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Song GB, Qin J, Luo Q, Shen XD, Yan RB, Cai SX. Adhesion of different cell cycle human hepatoma cells to endothelial cells and roles of integrin beta1. World J Gastroenterol. 2005;11:212-215. [PubMed] |
| 12. | Marconi A, Dallaglio K, Lotti R, Vaschieri C, Truzzi F, Fantini F, Pincelli C. Survivin identifies keratinocyte stem cells and is downregulated by anti-beta1 integrin during anoikis. Stem Cells. 2007;25:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Nakayama K, Kamihira S. Survivin an important determinant for prognosis in adult T-cell leukemia: a novel biomarker in practical hemato-oncology. Leuk Lymphoma. 2002;43:2249-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2342] [Cited by in RCA: 2383] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 15. | Yamamoto T, Tanigawa N. The role of survivin as a new target of diagnosis and treatment in human cancer. Med Electron Microsc. 2001;34:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 219] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med. 2001;7:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 324] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247-3257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 583] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21:2613-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 417] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58:1808-1812. [PubMed] |
| 21. | Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071-5074. [PubMed] |
| 22. | Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2873] [Cited by in RCA: 2942] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 24. | Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1385] [Cited by in RCA: 1438] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 25. | Wei MC. Bcl-2-related genes in lymphoid neoplasia. Int J Hematol. 2004;80:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Prives C, Hall PA. The p53 pathway. J Pathol. 1999;187:112-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Malerba I, Gribaldo L, Diodovich C, Carloni M, Meschini R, Bowe G, Collotta A. Induction of apoptosis and inhibition of telomerase activity in human bone marrow and HL-60 p53 null cells treated with anti-cancer drugs. Toxicol In Vitro. 2005;19:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Ji ZN, Ye WC, Liu GG, Hsiao WL. 23-Hydroxybetulinic acid-mediated apoptosis is accompanied by decreases in bcl-2 expression and telomerase activity in HL-60 Cells. Life Sci. 2002;72:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Coutts AS, La Thangue N. The p53 response during DNA damage: impact of transcriptional cofactors. Biochem Soc Symp. 2006;181-189. [PubMed] |
| 30. | Erster S, Moll UM. Stress-induced p53 runs a transcription-independent death program. Biochem Biophys Res Commun. 2005;331:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |