Published online Mar 21, 2007. doi: 10.3748/wjg.v13.i11.1666
Revised: December 29, 2006
Accepted: March 6, 2007
Published online: March 21, 2007
AIM: To evaluate the effect of pyrrolidine dithio-carbamate (PDTC; an NF-κB inhibitor) administered at low (50 mg/kg) and high (100 mg/kg) doses in suppressing colitis in mice with dextran sodium sulfate (DSS)-induced colitis.
METHODS: Mice were divided into a DSS-untreated group (normal group), DSS-treated control group, DSS+PDTC-treated groupI(low-dose group), and DSS+PDTC-treated groupII (high-dose group). In each group, the disease activity index score (DAI score), intestinal length, histological score, and the levels of activated NF-κB and inflammatory cytokines (IL-1β and TNF-α) in tissue were measured.
RESULTS: The DSS+PDTC-treated groupII exhibited suppression of shortening of intestinal length and reduction of DAI score. Activated NF-κB level and IL-1β and TNF-α levels were significantly lower in DSS+PDTC-treated groupII.
CONCLUSION: These findings suggest that PDTC is useful for the treatment of ulcerative colitis.
- Citation: Hirata I, Yasumoto S, Toshina K, Inoue T, Nishikawa T, Murano N, Murano M, Wang FY, Katsu KI. Evaluation of the effect of pyrrolidine dithiocarbamate in suppressing inflammation in mice with dextran sodium sulfate-induced colitis. World J Gastroenterol 2007; 13(11): 1666-1671
- URL: https://www.wjgnet.com/1007-9327/full/v13/i11/1666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i11.1666
Nuclear factor-κB (NF-κB) is a transcription activation factor that moves from the cytoplasm into the nucleus following appropriate extracellular signaling. It is thought to play roles in inflammation, immune reactions, carcinogenesis and apoptosis. NF-κB is composed of a family of genes with a high degree of homology. Five subtypes of NF-κB have been identified, including NF-κB 1 (p50), NF-κB 2 (p52), Rel A (p65), Rel B, and c-rel, which form hetero- or homo-dimers. Hetero-dimers of p65 and p50 are most abundant in cells. NF-κB is usually found in the cytoplasm conjugated to an inhibitory protein termed IκB. Phosphorylation of IκB by IκB kinase (IKK) following inflammatory signal transduction leads to degradation of IκB via proteosome, resulting in the transfer of NK-κB into the nucleus and its activation there[1].
Pyrrolidine dithiocarbamate (PDTC) is a NF-κB inhibitor. Various studies have been performed in attempts to suppress inflammation mediated by the NF-κB pathway[2,3]. Németh et al[4] reported that treatment of intestinal epithelial cells with PDTC in vitro suppressed the activity of NF-κB. All studies thus far reported, involving evaluation of the efficacy of PDTC in suppressing intestinal inflammation, were performed in vitro, and no such in vivo study has been reported. The present study was undertaken to evaluate the efficacy of intraperitoneally administered PDTC in suppressing inflammation in mice with dextran sodium sulfate (DSS)-induced colitis in vivo.
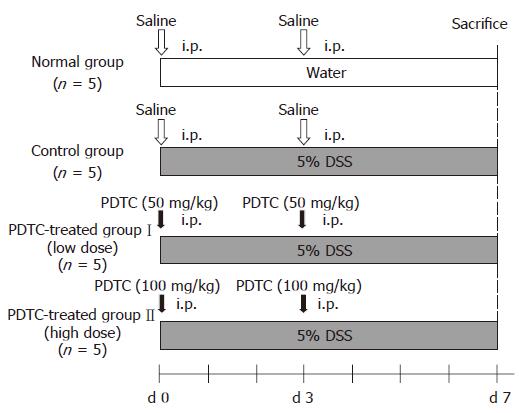
Six-week-old female BALB/c mice (SLC, Shizuoka) were used for this study. DSS with a molecular weight of 5000 (Nacalai Tesque, Kyoto) was dissolved in tap water to obtain 5% DSS solution. Mice were allowed free access to this solution as drinking water for 7 d to prepare a mouse model of DSS-induced colitis. PDTC (Sigma), dissolved in distilled water, was administered intraperitoneally to mice at dose levels of 100 and 50 mg/kg. Mice were divided into four groups. In the DSS-untreated group (normal group, n = 5), each mouse was allowed free access to tap water for 7 d. In the DSS-treated control group (control group, n = 5), each animal was allowed free access to 5% DSS solution, supplied as drinking water, for 7 d, and underwent intraperitoneal administration of physiological saline immediately before and 3 d after the start of DSS treatment. In the DSS+PDTC-treated groupI (low-dose group, n = 5), each animal was allowed free access to 5% DSS solution for 7 d and underwent intraperito-neal administration of PDTC (50 mg/kg) immediately before and 3 d after the start of DSS treatment. In the DSS+PDTC-treated group II (high-dose group, n = 5), each animal was allowed free access to 5% DSS solution for 7 d and underwent intraperitoneal administration of PDTC (100 mg/kg) immediately before and 3 d after the start of DSS treatment (Figure 1).
On the d 7 of the experiment, each animal was weighed to check for weight loss, and the appearance of feces and severity of bloody stool were also checked, followed by calculation of the DAI score according to the method reported by Murthy et al[5] (Table 1). Each mouse was then sacrificed and the large intestine was immediately removed for measurement of intestinal length and evaluation of intestinal shortening. Furthermore, the histological score of HE-stained specimens of the distal segment of the colon was determined in accordance with the method for scoring reported by Cooper et al[6] (Table 2).
| Score | Weight loss (%) | Stool consistency | Occult/grossbleeding |
| 0 | (-) | Normal | Normal |
| 1 | 1-5 | ||
| 2 | 6-10 | Loose | Guiac (+) |
| 3 | 11-15 | ||
| 4 | > 15 | Diarrhea | Gross bleeding |
| Grade 0 | Normal colonic mucosa | |
| Grade 1 | Loss of one-third of the crypts | |
| Grade 2 | Loss of two-third of the crypts | |
| Grade 3 | The lamina propria is covered with a single layer of epithelium and mild inflammatory cell infiltration is present | |
| Grade 4 | Erosions and marked inflammatory cell infiltration are present |
On the d 7 of the experiment, a protein extract from the distal segment of the intestine of each sacrificed mouse was obtained, using a PARIS Kit (Ambion). The levels of the inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in the extract were measured by ELISA, using the Quantkine Mouse IL-1β/IL-1F Immunoassay Kit and Mouse TNF-α/TNFSF1A Immunoassay Kit (R&D Systems, Minneapolis, MN, USA). The experiment was repeated on other mice in the same fashion as described above, except for the timing of sacrifice (the d 6 instead of the d 7 of the experiment). A nuclear extract from the distal segment of the intestine of each sacrificed mouse was obtained using a Nuclear/Cytosol Fractionation Kit (Biovision). The level of NF-κB in the extract was measured by ELISA, using a MercuryTM TransFactor Kit (NF-κB p65, BD Biosciences), to evaluate activation of NF-κB.
The data was analyzedsis using the t-test. mean ± SE values are presented for each parameter.
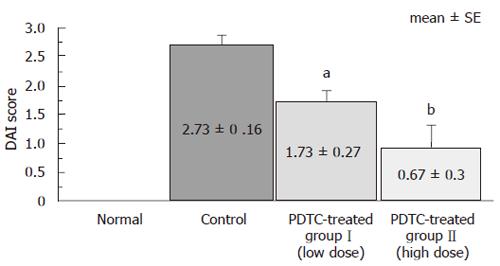
Compared to the control group, both DSS+PDTC-treated groupI and DSS+PDTC-treated group II exhibited marked reduction of weight loss, improvement in appear-ance of feces, and alleviation of bloody stool. DAI score, an indicator of the severity of intestinal inflammation, was 0 in the normal group, 2.73 ± 0.16 in the control group, 1.73 ± 0.27 in DSS+PDTC-treated groupI, and 0.67 ± 0.30 in DSS+PDTC-treated group II. Thus, significant suppression of inflammation was noted in DSS+PDTC-treated groupI and DSS+PDTC-treated group II compared to the control group (P < 0.05). Suppression was strongest in DSS+PDTC-treated group II (P < 0.01) (Figure 2).
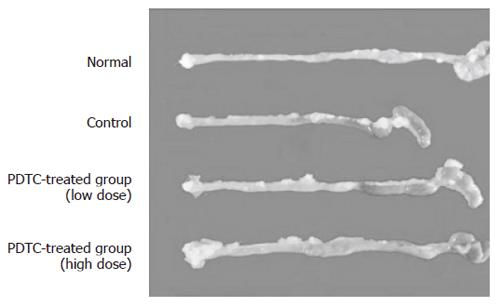
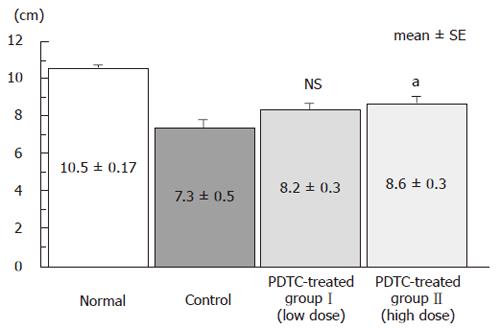
The effect of DSS on the severity of intestinal short-ening caused by intestinal inflammation was evaluated. Pictures of intestine from the normal group, the control group, and DSS+PDTC-treated groupsI and II are shown in Figure 3. Intestinal length was 10.5 ± 0.17 cm in the normal group, 7.3 ± 0.5 cm in the control group, 8.2 ± 0.3 cm in DSS+PDTC-treated groupI, and 8.6 ± 0.3 cm in DSS+PDTC-treated group II. Thus, shortening of the intestine was not significantly suppressed in the DSS+PDTC-treated groupI, but was in the DSS+PDTC-treated group II (P < 0.05) (Figure 4).
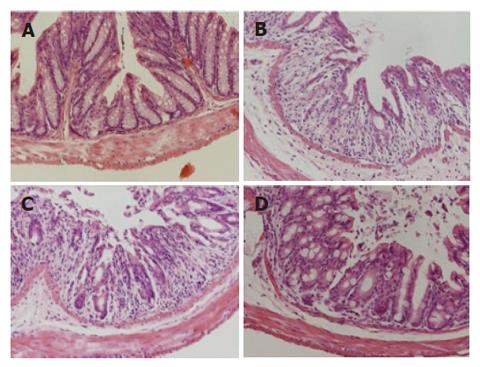
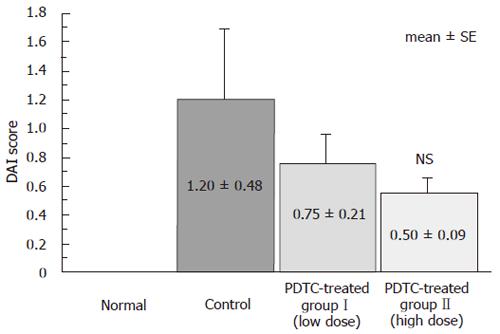
The histological score for the distal segment of the colon was 0 in the normal group, 1.20 ± 0.48 in the control group, 0.75 ± 0.21 in the DSS+PDTC-treated groupI, and 0.50 ± 0.09 in the DSS+PDTC-treated group II. Compared to the normal group (Figure 5A), the control group exhibited marked erosion of the lamina propria mucosae, disappearance of glandular epithelium, inflammatory cell infiltration, and other related findings (Figure 5B). In the DSS+PDTC-treated groupI (Figure 5C), the findings of evaluation of inflammation did not differ markedly from those in the control group. In the DSS+PDTC-treated group II (Figure 5D), erosion, disappearance of glandular epithelium, inflammatory cell infiltration, and other abnormalities tended to be less severe than those in the control group, although none of these differences was statistically significant (Figure 6).
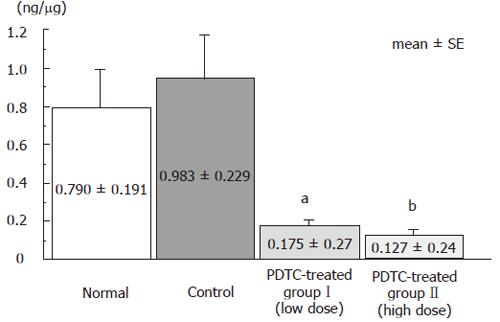
NF-κB levels in nuclear extract from distal colonic tissue were compared among groups. The level was 0.790 ± 0.191 ng/μg in the normal group, 0.938 ± 0.229 ng/μg in the control group, 0.175 ± 0.27 ng/μg in the DSS+PDTC-treated groupI, and 0.127 ± 0.24 ng/μg in the DSS+PDTC-treated group II. This parameter was thus slightly higher in the control group than in the normal group, and significantly lower in the DSS+PDTC-treated groupI (P < 0.05) and the DSS+PDTC-treated group II (P < 0.01). These findings suggest that treatment with PDTC suppressed activation of NF-κB (Figure 7).
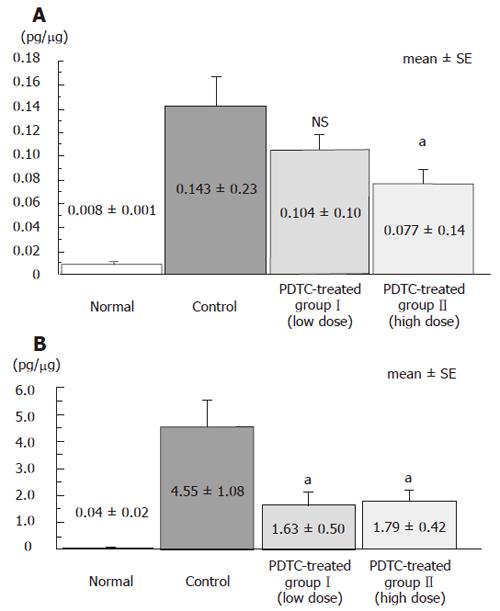
Levels of inflammatory cytokines IL-1β and TNF-α in distal intestinal tissue were compared among groups. IL-1β level was 0.008 ± 0.003 pg/μg in the normal group, 0.143 ± 0.52 pg/μg in the control group, 0.104 ± 0.32 pg/μg in the DSS+PDTC-treated groupI, and 0.077 ± 0.23 pg/μg in the DSS+PDTC-treated group II. Thus, this parameter tended to be lower in the DSS+PDTC-treated groupI than in the control group, although this difference was not statistically significant. However, this parameter was significantly lower in the DSS + PDTC treated group II (P < 0.05) than in the control group (Figure 8A). TNF-α level was 0.04 ± 0.06 pg/μg in the normal group, 4.55 ± 2.41 pg/μg in the control group, 1.63 ± 1.12 pg/μg in the DSS + PDTC treated groupI, and 1.79 ± 0.94 pg/μg in the DSS+PDTC-treated group II. Thus, this parameter was significantly lower in the DSS + PDTC treated groupI and the DSS+PDTC-treated group II than in the control group (P < 0.05) (Figure 8B).
Ulcerative colitis is an inflammatory bowel disease whose for which the etiology has not yet been fully clarified. Many studies of it have been carried out using animal models of enterocolitis. Numerous reports have been published concerning experiments using animal models of ulcerative colitis, i.e. rodent with DSS-induced colitis[7-9].
It is thought that NF-κB is associated with the exp-ression of various cytokines, chemokines, and adhesion factors in inflammation. NF-κB p65 protein has been reported to exhibit increased expression in nuclear extracts from local mucosal tissue specimens of patients with inflammatory bowel disease[10,11]. Recently reports have been published to evaluate the inflammation suppressing effect of antisense oligonucleotide for NF-κB (p65) administered to mice with TNBS-induced enteritis (a model of Crohn’s disease)[12] and mice with DSS-induced colitis (a model of ulcerative colitis)[13] or ischemia-reperfusion injury of the small intestine[14].
It has been reported that NF-κB binds to the DNA sequence termed “κB sequence” and induces expression of the genes regulated by this sequence. NF-κB is usually present in an inactive form, bound to IκB, within the cytoplasm. If IκB is detached, NF-κB can move into the nucleus to become activated. Of the IκB family members, IκB-α has recently been shown to play roles in the signaling triggering the entry of κB into the nucleus[15]. PDTC is a kind of antioxidants known to inhibit NF-κB. PDTC has been reported to suppress NF-κB activity more powerfully than any other drug of the dithiocarbamate family. Cuzzocrea et al[17] reported that PDTC suppresses the transfer of NF-κB from the cytoplasm into the nucleus by inhibition of the IκB-α degradation[16].
In the present study, BALB/c mice were treated with 5% DSS solution to induce acute colitis. DAI score was markedly higher in the control group than in the normal group. Histologically, the rectum of the control group exhibited marked inflammatory cell infiltration and erosion. As in a previous study using rats with DSS-induced colitis[18], the present study revealed marked increases in IL-1β and TNF-α, known to produce pri-marily by activated macrophages in the control group. In the DSS+PDTC-treated group II (mice intraperitoneally administered PDTC at a high dose, 100 mg/kg), suppression of intestinal shortening and improvement of DAI score were noted, accompanied by reduction of inflammatory cytokines IL-1β and TNF-α. Suppression of inflammation was most marked in DSS+PDTC-treated group II, in which NF-κB level in rectal nucleus extract was lower than in any other group. These findings demonstrate that treatment with PDTC suppresses the activity of NF-κB in the intestine, and suggest that treatment with PDTC reduces intestinal NF-κB activity and suppresses the production of IL-1β and TNF-α, resulting in alleviation of DSS-induced colitis.
However, on histopathological examination, no sig-nificant suppression of inflammation was noted in the DSS+PDTC-treated groupI (low dose) or the DSS+PDTC-treated group II (high dose). It has been reported that NF-κB is associated with cyclin D during the cell cycle and that p65/p50 hetero-dimer (a NF-κB molecule) binds to the NF-κB binding site of the cyclin D promoter, possibly resulting in appropriate regulation of cyclin D and triggering of the start of the cell cycle[19,20]. This indicates that suppression of NF-κB with PDTC will suppress progression of the cell cycle, resulting in delay of cell proliferation and tissue repair. Thus, in the present study, in which inflammation was not satisfactorily controlled, the mucosal damage arising from inflammation was found not to have been alleviated when examined histologically, because suppression of NF-κB had suppressed regene-ration of the damaged mucosa.
These findings suggest that suppression of NF-κB activity by PDTC can delay the healing of mucosal tissue defects (erosions or ulcers) arising from inflammation, but that it can strongly suppress the expression of inf-lammatory cytokines (IL-1β and TNF-α), resulting in significant alleviation of colitis.
We conclude that PDTC is promising as a drug cli-nically useful for the treatment of ulcerative colitis.
In inflammatory bowel disease, the inflammatory cytokines IL-1β and TNF-α play important roles in inflammatory reactions in the intestinal mucosa. NF-κB is one of the factors that induces expression of these inflammatory cytokines. The present study was aimed to evaluate the effect of NF-κB inhibitor PDTC administered at low (50 mg/kg) and high (100 mg/kg) doses in suppressing DSS-induced colitis in mice.
Various studies have been performed in attempts to sup-press inflammation mediated by the NF-κB pathway. Németh et al reported that treatment of intestinal epithelial cells with PDTC in vitro suppressed the activity of NF-κB. All studies thus far reported, involving evaluation of the efficacy of PDTC in suppressing intestinal inflammation, were performed in vitro, and no such in vivo study has been reported. The present study was undertaken to evaluate the efficacy of intraperitoneally administered PDTC in suppressing inflammation in mice with DSS-induced colitis in vivo.
These findings suggest that suppression of NF-κB activity by PDTC can delay the healing of mucosal tissue defects (erosions or ulcers) arising from inflammation, but that it can strongly suppress the expression of inflammatory cytokines (IL-1β and TNF-α), resulting in significant alleviation of colitis.
Therefore, PDTC may be promising as a drug clinically useful for the treatment of ulcerative colitis.
Pyrrolidine dithiocarbamate (PDTC) is a NF-κB inhibitor. Various studies have been performed in attempts to suppress inflammation mediated by the NF-κB pathway. Nuclear factor-κB (NF-κB) is a transcription activation factor which moves from the cytoplasm into the nucleus following appropriate extracellular signaling. It is thou-ght to play roles in inflammation, immune reactions, carcinogenesis and apoptosis.
This study is well constructed and well documented. They clearly presented the effects of PDTC on DSS-induced colitis in mice.
S- Editor Liu Y L- Editor Alpini GD E- Editor Liu Y
| 1. | Israël A. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 2000;10:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 309] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Long SM, Laubach VE, Tribble CG, Kaza AK, Fiser SM, Cassada DC, Kern JA, Kron IL. Pyrrolidine dithiocarbamate reduces lung reperfusion injury. J Surg Res. 2003;112:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Bruck R, Aeed H, Schey R, Matas Z, Reifen R, Zaiger G, Hochman A, Avni Y. Pyrrolidine dithiocarbamate protects against thioacetamide-induced fulminant hepatic failure in rats. J Hepatol. 2002;36:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Németh ZH, Deitch EA, Szabó C, Haskó G. Pyrrolidinedithiocarbamate inhibits NF-kappaB activation and IL-8 production in intestinal epithelial cells. Immunol Lett. 2003;85:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 446] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 7. | Cheon JH, Kim JS, Kim JM, Kim N, Jung HC, Song IS. Plant sterol guggulsterone inhibits nuclear factor-kappaB signaling in intestinal epithelial cells by blocking IkappaB kinase and ameliorates acute murine colitis. Inflamm Bowel Dis. 2006;12:1152-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. [PubMed] |
| 9. | Kimura I, Nagahama S, Kawasaki M, Kamiya A, Kataoka M. Study on the experimental ulcerative colitis (UC) model induced by dextran sulfate sodium (DSS) in rats (2). Nihon Yakurigaku Zasshi. 1995;105:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 580] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 11. | Neurath MF. Pathogenesis of inflammatory bowel disease: transcription factors in the spotlight. Gut. 1998;42:458-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Neurath MF, Pettersson S, Meyer zum Büschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 631] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 13. | Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, Sasaki S, Saitoh O, Katsu K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 251] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Mallick IH, Yang WX, Winslet MC, Seifalian AM. Pyrrolidine dithiocarbamate reduces ischemia-reperfusion injury of the small intestine. World J Gastroenterol. 2005;11:7308-7313. [PubMed] |
| 15. | Malek S, Chen Y, Huxford T, Ghosh G. IkappaBbeta, but not IkappaBalpha, functions as a classical cytoplasmic inhibitor of NF-kappaB dimers by masking both NF-kappaB nuclear localization sequences in resting cells. J Biol Chem. 2001;276:45225-45235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Topping RJ, Jones MM. Optimal dithiocarbamate structure for immunomodulator action. Med Hypotheses. 1988;27:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Shintani N, Nakajima T, Nakakubo H, Nagai H, Kagitani Y, Takizawa H, Asakura H. Intravenous immunoglobulin (IVIG) treatment of experimental colitis induced by dextran sulfate sodium in rats. Clin Exp Immunol. 1997;108:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690-2698. [PubMed] |
| 20. | Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785-5799. [PubMed] |