INTRODUCTION
Chylous ascites is a rare clinical manifestation characterized by ascitic chylomicrons resulted from mechanical obstruction of or leakage from the lymphatic channel. chronic disorders, especially malignancies, accounting for most cases of chylous ascites. Mesenteric fibromatosis is a rare benign mesenteric tumor, characterized by infiltrative growth and high rates of recurrence[1]. There is no case report about mesenteric fibromatosis with chylous ascites, which is difficult to diagnose and differentiate from other tumors, thereby leading to diagnostic as well as therapeutic dilemmas[2]. Even when the diagnosis is well-established, mesenteric fibromatosis with chylous ascites still presents a management challenge for the surgeon to make an optimal therapeutic decision for the patient suffering from this disease[3,4].
CASE REPORT
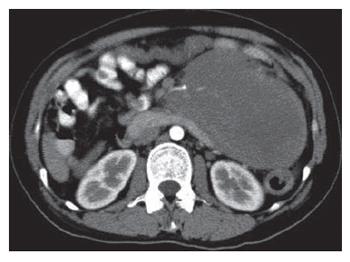
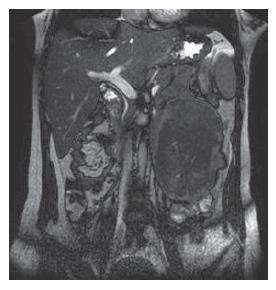
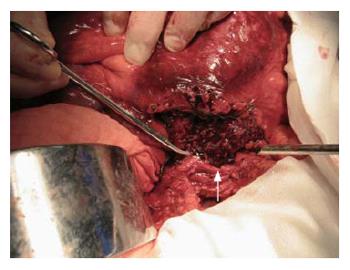
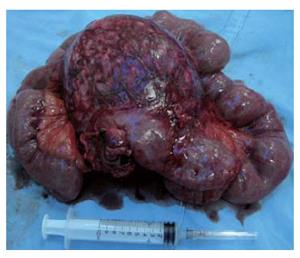
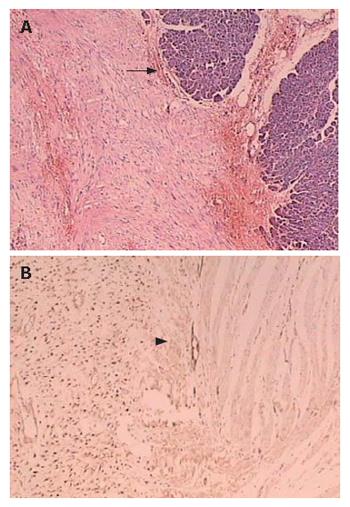
A 28-year old woman was admitted 1 mo ago for a caesarean section operation and a healthy boy was born. However, 250 mL chylous ascites was found in her abdominal cavity during the operation. Sonographic examination detected a tumor in the left upper quadrant of the abdomen after her delivery. Multidetector spiral computed tomography (MDCT) implied that a mass was arising from the mesentery, semi-surrounding the superior mesenteric artery (SMA) and superior mesenteric vein (SMV) (Figure 1). Magnetic resonance imaging (MRI) revealed a tumor with low to intermediate mixed signal intensity in the lesion’s periphery on T1 weighted images and bands of high signal intensity in the central region of the lesion on T2 weighted images (Figure 2). Digital subtraction angiography (DSA) demonstrated that the tumor’s blood supply was poor and the endangium of SMA was smooth without lumen stenosis. A tumor was found about 6 cm beneath the suspensory ligament of duodenum during another laparotomy. The mesenteric vessels were almost semi-surrounded and the pancreas was adhered to the tumor tissue (Figure 3). The entire tumor with a loop of 86 cm jejunum was resected completely. The tumor measured 12.2 cm × 11.5 cm × 8.5 cm and was closely related to the bowel wall (Figure 4). The cut surface was tan-colored, whorled and firm, without necrosis, cystic change or haemorrhage. Microscopy of the resected slices showed loosely and haphazardly arranged spindle cells with bland, oval nuclei and minimal cytoplasm. There were also plump spindle cells with tapering ends containing oval, vesicular nuclei and a moderate amount of eosinophilic cytoplasm. The pancreas and bowel wall were infiltrated by the mass (Figure 5A). Subsequent immunohistochemistry analysis had the following results: CD117 (-), CD34 (-), focal ACT (+), DES (-), S-100 (+++), β-catenin (-) (Figure 5B). A 12-mo follow-up period revealed a good condition of the patient.
Figure 1 Contrast-enhanced axial scan of MDCT revealing a heterogeneous mesenteric huge soft tissue mass, which displaces the SMA.
Figure 2 Coronal MRI image demonstrating a tumor with low to intermediate mixed signal intensity in the lesion's periphery and low signal intensity in the central region of the lesion on T2 weighted images.
Figure 3 Semi-surrounding SMA and SMV (arrow) demonstrated during operation.
Figure 4 Resected small bowel with a well-circumscribed firm mass exhibiting an expanding growth pattern.
Figure 5 Tumoral lesion consisted of spindle cells growing in sweeping fascicles, with eosinophilic cytoplasm and occasional focal infiltration of plump nuclei in pancreas (arrow) (A) and bowel wall (arrow head) (B).
Most of the tumor cells showed immunoreactivity for β-catenin (+++).
DISCUSSION
Chylous ascites is brought about by lymph leaking into the abdominal cavity. Although chylous ascites sometimes may be caused by trauma, abdominal surgery, tuberculosis or other peritoneal infections, it is usually found as a symptom of tumor especially malignancies. There are few cases reporting chylous ascites during pregnancy, mainly caused by acute pancreatitis, small intestine volvulus and other conditions[5]. However, the symptoms and signs of an abdominal mass that grows during pregnancy are not easy to detect and identify. Aggressive mesenteric fibromatosis during pregnancy was rarely reported and aggressive mesenteric fibromatosis associated with chylous ascites has never been reported. Most cases of aggressive mesenteric fibromatosis reported in elderly individuals, are usually associated with familial polyposis coli, previous trauma, and hormonal imbalance[6]. Our case was a woman suffering from this disease during her pregnancy. The onset of the disease may be due to hormonal imbalance. The high level of serum lutin and estrin may induce the initiation of the disease in her gestation period[7]. Due to compression of the alveus ampullescens by the mass at the root of mesentery, lymphatic return was blocked as a result of chylous ascites formation.
Surgery provides good results in limiting the disease and non-surgical modalities are applied to cases with unresectable or residual lesions. In the preoperative evaluation of mesenteric fibromatosis, a careful investigation of its clinicopathological features is very important in selecting an appropriate approach to the surgical candidates[8]. Mesenteric fibromatosis is a rare, benign fibrous lesion originating from the bowel mesentery or the retroperitoneum. Characteristics its biological behavior present in the intermediate between benign fibrous tissue proliferation and fibrosarcoma. Fibromatosis has a typical inclination towards involving the visceral abdominal structures in its growth, and tends to recur but does not metastasize[9]. During the laparotomy, our case revealed a large mesenteric mass arising from the mesentery. The lesion was surrounded by a segment of intestinal canal and adhered to the pancreas. Semi-enclosed by the tumor tissue, the mesenteric vessels were displaced from their normal position. The tumors were excised together with a segment of jejunum.
Radiological studies play a significant role in establishing the diagnosis, working out the operation plan and evaluating the situation in follow-up of mesenteric fibromatosis. MDCT has an advantage of characterizing the mass in showing the homogeneity of attenuation, exhibiting slight enhancement after contrast medium administration. MDCT also provides a concise evaluation of the extension of the lesion. Images of reconstructed sagittal, coronal, or curved planes are very useful in most cases[10]. MRI may show characteristic features in prominent low to intermediate signal intensity and bands of low signal intensity representing highly collagenized tissue. However, fibromatoses with less collagen and more cellularity may have nonspecific high signal intensity on T2-weighted images[11]. DSA clearly depicts the bole and branch of SMA, which extend from the root of the mesentery towards the pelvis. Furthermore, it provides a clear description of the SMA and SMV lumen condition, confirming whether the main vasculature has been infiltrated[12]. Such unique imaging approaches may ultimately improve lesion detection, characterization, and surgical planning of the case.
Immunohistochemical analysis is very important for distinguishing mesenteric fibromatosis from gastrointestinal stromal tumor, which is clinically important in the Gleevec era[13]. Pathologists must differentiate this condition from a host of fibroblastic and myofibroblastic lesions or from smooth muscle neoplasms. Almost all-deep fibromatoses have somatic beta-catenin or adenomatous polyposis coli gene mutations leading to intranuclear accumulation of beta-catenin[14]. Since low-grade sarcomas in general lack beta-catenin and reactive proliferations are not expected to have it, nuclear beta-catenin expression would be detected in deep fibromatoses but absent in other entities in the differential diagnosis[15]. Beta-catenin immunohistochemistry separates deep fibromatosis from entities in the differential diagnosis, a finding that can be exploited for diagnosis[13,16]. Most fibromatoses have diffused nuclear staining and focal staining may be occasionally seen.
In conclusion, aggressive mesenteric fibromatosis with chylous ascites during pregnancy has never been reported, and it is not easy to make a diagnosis before and after operation. Radiological studies have a great value in designing management of the disease. In preoperative evaluation of mesenteric fibromatosis, its clinicopathological features are very important for working out an appropriate operation plan for surgical candidates. Immunohistochemical analysis of specimens offers the final diagnosis of the disease that may be confused with other kinds of tumor. Our report highlights the need of combining clinicopathological, radiological, and immunohistochemistry analysis in management of the disease.
ACKNOWLEDGMENTS
The authors thank Dr Jian-Chun Cai and Dr Jun-Ming Xu, Department of Oncosurgery, the First Hospital of Xiamen, Fujian Medical University, for their assistance in data collection.