Published online Mar 14, 2007. doi: 10.3748/wjg.v13.i10.1595
Revised: December 15, 2006
Accepted: January 25, 2007
Published online: March 14, 2007
AIM: To determine the association between H pylori infection and serum ghrelin levels in patients without atrophic gastritis.
METHODS: Fifty consecutive patients (24 males and 26 females) with either H pylori-positive gastritis (n = 34) or H pylori-negative gastritis (n = 16) with normal gastric acid secretion determined by 24-h pHmetry and without atrophic gastritis in histopathology were enrolled in this study. Thirty-four H pylori-infected patients were treated with triple therapy consisting of a daily regimen of 30 mg lansoprazole bid, 1 g amoxicillin bid and 500 mg clarithromycin bid for 14 d, followed by an additional 4 wk of 30 mg lansoprazol treatment. H pylori infection was eradicated in 23 of 34 (67.6%) patients. H pylori-positive patients were given eradication therapy. Gastric acidity was determined via intragastric pH catethers. Serum ghrelin was measured by radioimmunoassay (RIA).
RESULTS: There was no signifficant difference in plasma ghrelin levels between H pylori-positive and H pylori-negative groups (81.10 ± 162.66 ng/L vs 76.51 ± 122.94 ng/L). In addition, there was no significant difference in plasma ghrelin levels and gastric acidity levels measured before and 3 mo after the eradication therapy.
CONCLUSION: H pylori infection does not influence ghrelin secretion in patients with chronic gastritis without atrophic gastritis.
-
Citation: Cindoruk M, Yetkin I, Deger SM, Karakan T, Kan E, Unal S. Influence of
H pylori on plasma ghrelin in patients without atrophic gastritis. World J Gastroenterol 2007; 13(10): 1595-1598 - URL: https://www.wjgnet.com/1007-9327/full/v13/i10/1595.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i10.1595
H pylori is a Gram-negative bacteria that colonizes on gastric mucosa and is the important etiological agent for gastric ulcer and carcinomas[1]. However, it has been reported that long-term persistent H pylori infection leads to atrophic gastritis, which increases the risk of gastric adenocarcinomas[2]. The rate of peptic ulcer in H pylori-infected population is approximately 3% in USA and 25% in Japan[3]. The rate of H pylori infection is 50% in worldwide[4]. The H pylori prevalence varies according to the socioeconomic status. The prevalence of H. pylori is approximately 67.6%-81.3% in Turkey[5]. For developing clinical disease, host genetics, host immune response, and bacterial virulence factors appear to play critical roles[6].
Ghrelin is a recently discovered growth hormone-releasing peptide which is mostly produced by gastric X-like cells[7] and it has been shown to increase food intake and body mass gain, alters gastric motility and acid secretion[8]. Ghrelin is also released from the small intestine, kidney, hypothalamus, pancreas, placenta and pituitary gland[9]. Plasma ghrelin concentrations increase before meals and decrease postprandially. Ghrelin is the first circulating hormone shown to promote feeding and adiposity following systemic administration in animals[10] and it has been demonstrated that ghrelin potently stimulates appetite and food intake in man[8]. Animal studies have shown that administration of ghrelin into intracerebroventricular compartment or via peripheral vein decreases gastric acid secretion in rats[11,12].
Gastric acid secretion is affected by nervous system, several hormones like ghrelin and somatostatin as well as H pylori infection[13]. There are a few data in the literature about the relationship between H pylori and ghrelin. Masaoka et al[14] reported on a case of 31-year-old man with H pylori-positive gastritis. In this case, the plasma levels of total and active ghrelin showed no marked changes after successful eradication therapy on the long-term follow-up[14]. Several other studies demonstrated that in H pylori-positive individuals, the plasma ghrelin levels were significantly lower compared with the patients without H pylori infection[15,16]. According to these reports, H pylori infection decreased plasma ghrelin concentrations and ghrelin levels were increased after eradication[17]. On the contrary, Gokcel et al[18] reported H pylori infection had no effect on plasma ghrelin concentration. In the aforementioned studies, patients with decreased plasma ghrelin levels and H pylori infection were suffering from atrophic gastritis, leading to gastric hypoacidity. The effect of H pylori infection on gastric acidity independent of atrophic gastritis is not thoroughly investigated. For this reason, we aimed to determine the effect of H pylori infection on plasma ghrelin levels in patients with normal gastric acidity and without atrophic gastritis.
Fifty consecutive patients (24 males and 26 females) with normal 24-h pHmetry analysis were enrolled in the present study. All patients were performed upper gastrointestinal endoscopy and biopsy. In accordance with the Helsinki declaration,written informed consent was taken from all patients. The study was approved by Local Ethics Committee. The inclusion criteria were normal 24-h gastric pHmetry analysis and absence of gastric atrophy. Exclusion criteria were: age < 18 or > 70 year, abnormal gastric acidity, presence of atrophic gastritis in the histopathological evaluation, history of prior H pylori eradication therapy, acid suppressive therapy in the last 6 wk, prior gastrectomy, gastric cancer, diabetes mellitus, chronic renal disease, liver disease, drug addiction, alcohol abuse, pregnancy, and body mass index (BMI) > 30 kg/m2. Fifty patients (24 males, 26 females) with either H pylori-positive gastritis (n = 34) or H pylori-negative gastritis (n = 16) were enrolled.
H pylori was detected by histological evaluation of gastric biopsy samples (two biopsies from antrum and corpus) and 13C-urea breath test. Any positive result was accepted as presence of H pylori infection.
Thirty-four H pylori-infected patients were treated with triple therapy consisting of a daily regimen of 30 mg Lansoprazole bid, 1 g amoxicillin bid and 500 mg clarithromycin bid for 14 d, followed by an additional 4 wk of 30 mg lansoprazol treatment. H pylori infection was eradicated in 23 out of 34 (67.6%) patients. Body mass and height were measured while subjects wore light clothing without shoes. A complete medical history and physical examination were carried out to exclude endocrine disorder, gastrointestinal disease and operations or any other condition known to affect endocrine and gastric function.
H pylori eradication was re-evaluated six weeks after completion of the therapy by endoscopic biopsies (2 samples from antrum and corpus) and 13C-urea breath test. Any positive test was regarded as therapy failure. Endoscopy was performed after overnight fasting and two specimens were taken from intact mucosa in the gastric antrum and corpus. The specimens were stained with hematoxylin and eosin and Giemsa to demonstrate H pylori. Gastritis was described according to the Sydney Classification[19].
Patients with and without H pylori infection were evaluated with 24-h ambulatory pH monitoring. Patients with H pylori infection had a control pHmetry 3 mo after the cure of H pylori infection, in order to rule out any late effects of H pylori eradication on gastric acidity. After overnight fast, in our motility laboratory pH electrode with two channels was inserted through the nose into the esophagus. The pH electrode was positioned 10 cm below the lower esophageal sphincter. The pH signal was recorded by Digitrapper MK III (Medtronics Functional Diagnostic). Gastric acidity levels were assessed by monitoring gastric pH during 24 h and the time with pH below 4 was calculated to determine the individual’s gastric acidity levels.
On the day of endoscopy, blood samples were taken between 8:00 am and 10:00 am after an overnight fast, transferred into tubes containing EDTA and centrifuged, then plasma was separated and stored at -80°C until assay. Blood samples were taken before and 3 mo after the cure of H pylori infection. Plasma ghrelin concentrations were measured by radioimmunoassay (RIA) at Gazi University Hormone Research Laboratory.
Data were expressed as means ± SE. Fisher-exact test, Cruscall Wallis, Mann-Whitney U test, Wilcoxon tests and correlation analysis were used to determine significant differences between the values in various groups of patients. P < 0.05 was considered statistically significant. Data were analyzed using SPSS for Windows (version 11.0; SPSS).
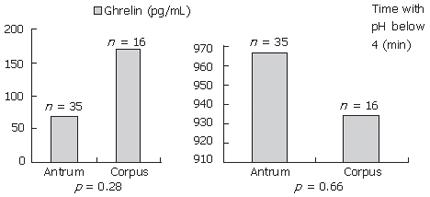
The study population included 26 females and 24 males with normal gastric acidity levels evaluated with 24-h ambulatory pH monitoring (Table 1). None of these patients had atrophic gastritis in the histological examination. Thirty-five of the patients had antrum predominant and 16 of the patients had corpus predominant gastritis. There were no significant differences in the baseline characteristics of the patients. Mean fasting serum ghrelin level was higher in the patients with higher BMI (> 25 kg/m2) than the patients with BMI < 25 kg/m2 (99.0 ± 176.3 ng/L vs 60.4 ± 111.2 ng/L, P = 0.04; Table 2). Age and gender had no effect on ghrelin levels. There were no significant differences in mean plasma ghrelin levels between H pylori-positive and H pylori-negative groups (81.10 ± 162.66 ng/L vs 76.51 ± 122.94 ng/L). Plasma ghrelin levels before and after eradication of H pylori infection were also similar in H pylori-positive patients (78.34 ± 101.45 ng/L vs 88.67 ± 111.21 ng/L). When compared between antrum predominant and corpus predominant group, plasma ghrelin levels did not differ significantly (Figure 1).
| Clinical features | Male (n = 24) | Female (n = 26) |
| Age (yr) | 40.9 ± 10.7 | 41.2 ± 14.5 |
| BMI (kg/m2) | 28.7 ± 0.9 | 23.9 ± 3.4 |
| H pylori, n (%) | ||
| Positive | 19 (77.8) | 16 (61.5) |
| Negative | 5 (22.2) | 10 (28.5) |
| Topoghrapy of gastritis, n (%) | ||
| Antrum | 20 (83.3) | 15 (86.7) |
| Corpus | 4 (16.7) | 11 (13.3) |
H pylori infection was cured in 23 patients who received eradication therapy. There was no significant difference in plasma ghrelin levels and gastric acidity measured before and after treatment.
Ghrelin is synthesized from gastric mucosa[1] and also released from the small and large intestine, hypothalamus, and pancreas[16]. Plasma ghrelin levels change in a narrow spectrum because of the secretion from other tissues. It has been shown in controlled studies that ghrelin increases food intake and eating/appetite and leads to increased body mass[20]. In the present study, we found that mean fasting serum ghrelin level was higher in the patients with BMI > 25 kg/m2 than the patients with BMI < 25 kg/m2.
To date, there are few studies discussing the association between ghrelin level, which is produced from the gastric oxyntic cells, and H pylori infection. In addition, available data in the literature have contradiction regarding the association between ghrelin level and H pylori infection. Nwokolo et al[21] reported that after H pylori eradication therapy, plasma ghrelin levels significantly increased, hypothesizing that this elevation in plasma ghrelin levels led to obesity by raising appetite. Ghrelin production was assumed to be reduced as a result of H pylori infection. H pylori infection led to chronic gastritis and atrophy, thereby reducing number of oxynthic cells, the main cells producing ghrelin. In a case of 31-year-old man with H pylori-positive gastritis, the plasma levels of total and active ghrelin showed no marked changes after successful eradication therapy on the long-term follow-up[14]. Suzuki et al[22] investigated gastric and plasma ghrelin dynamics in H pylori-infected Mongolian gerbils. Although expressions of preproghrelin mRNA in the gastric mucosa and total ghrelin were reduced after 17 and 23 wk of H pylori infection, a compensatory increase in ghrelin secretion was noted at wk 17. Isomoto et al[15] reported variable plasma ghrelin levels in 257 patients with various upper gastrointestinal diseases. In this study, the patients with chronic gastritis had lowest level of ghrelin than the patients with acute gastritis, duodenal or gastric ulcer, gastric cancer, benign gastric polyp, reflux esophagitis and normal gastric mucosa. Pepsinogen I/II ratio was found to be significantly correlated with plasma ghrelin levels, especially in H pylori-positive patients. These findings indicate the significant effect of gastric atrophy on impaired ghrelin secretion in H pylori-infected patients. The improvement of gastric acidity and ghrelin levels may be parallel to the improvement of atrophic gastritis in previous reports. Gokcel et al[18] failed to demonstrate any association between H pylori and plasma ghrelin levels. However, in this study, the authors did not describe the degree of gastric atrophy in the study group. For this reason, we are not able to comment about gastric atrophy-induced impaired secretion of ghrelin in this study. H pylori species leading to atrophic gastritis, such as cag A, is less prevalent in Turkey[23]. For this reason, it is probable that geographical differences might influence the variable ghrelin levels after H pylori eradication.
In our study, we enrolled patients without atrophic gastritis and with normal gastric acidity. In this special subgroup of patients, H pylori infection and its eradication had no impact on the serum levels of ghrelin. A study, which aimed to determine plasma ghrelin level variations according to topographical distribution of gastritis, showed that in the antrum dominant gastritis, ghrelin levels were lower than the corpus predominant gastritis[15]. In contrast, we did not observe any significant difference between the antrum dominant and corpus dominant gastritis for plasma ghrelin levels.
Although other studies preferred at least 6 mo of duration to accurately assess the relevant gastric acid recovery, previous studies have shown that gastric acid secretion changes rapidly, even after one month, in patients with duodenal ulcer[24,25]. For this reason, we checked the gastric acidity levels 3 mo after H pylori eradication.
In conclusion, H pylori infection has no effect on plasma ghrelin concentration in Turkish population without atrophic gastritis.
Many studies demonstrated that in H pylori-positive individuals, the plasma ghrelin levels were significantly lower than that in patients without H pylori infection. H pylori infection decreased plasma ghrelin concentrations and ghrelin levels were increased after H pylori eradication. However, none of the studies evaluated the ghrelin-H pylori relationship in the patients without atrophic gastritis.
Previous studies on ghrelin and H pylori infection were performed on heterogenous group of patients. Ghrelin levels in various gastric diseases, including duodenal ulcer, gastric ulcer, reflux disease or bile acid reflux should be investigated as a seperate entity.
In this study, it has been shown that patients without atrophic gastritis and with normal gastric acidity had similar serum ghrelin levels irrespective of their H pylori status.
Atrophic gastritis and gastric hypoacidity are main factors affecting serum ghrelin level changes associated with H pylori infection. For this reason, early detection and eradication of H pylori is crucial for the prevention of irreversible changes of gastric mucosa.
To concisely and accurately describe, define or explain the specific, unique terms that are not familiar to majority of the readers, but are essential for the readers to understand the article.
The article provides relevant data about the role of H pylori infection in ghrelin metabolism, a growth hormone-releasing peptide mostly produced by gastric X-like cells and shown to increase food intake and weight gain among other minor gastrointestinal effects.
S- Editor Wang J L- Editor Kumar M E- Editor Che YB
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3265] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 2. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Schlemper RJ, van der Werf SD, Biemond I, Lamers CB. Seroepidemiology of gastritis in Japanese and Dutch male employees with and without ulcer disease. Eur J Gastroenterol Hepatol. 1996;8:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1234] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 5. | Us D, Hasçelik G. Seroprevalence of Helicobacter pylori infection in an Asymptomatic Turkish population. J Infect. 1998;37:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Figueiredo C, Machado JC, Yamaoka Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter. 2005;10 Suppl 1:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Murray CD, Kamm MA, Bloom SR, Emmanuel AV. Ghrelin for the gastroenterologist: history and potential. Gastroenterology. 2003;125:1492-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1284] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 9. | Otto Buczkowska E. The role of ghrelin in the regulation of energy homeostasis. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2005;11:39-42. [PubMed] |
| 10. | Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325-4328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 834] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 11. | Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 265] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, Hosoda H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 619] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 13. | Kaneko H, Konagaya T, Kusugami K. Helicobacter pylori and gut hormones. J Gastroenterol. 2002;37:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Masaoka T, Suzuki H, Imaeda H, Hosoda H, Ohara T, Morishita T, Ishii H, Kangawa K, Hibi T. Long-term strict monitoring of plasma ghrelin and other serological markers of gastric diseases after Helicobacter pylori eradication. Hepatogastroenterology. 2005;52:1-4. [PubMed] |
| 15. | Isomoto H, Ueno H, Nishi Y, Yasutake T, Tanaka K, Kawano N, Ohnita K, Mizuta Y, Inoue K, Nakazato M. Circulating ghrelin levels in patients with various upper gastrointestinal diseases. Dig Dis Sci. 2005;50:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Isomoto H, Nakazato M, Ueno H, Date Y, Nishi Y, Mukae H, Mizuta Y, Ohtsuru A, Yamashita S, Kohno S. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am J Med. 2004;117:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, Kishida T, Fukuda Y, Sugisaki Y, Sakamoto C. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol. 2004;99:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Gokcel A, Gumurdulu Y, Kayaselcuk F, Serin E, Ozer B, Ozsahin AK, Guvener N. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol. 2003;148:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3555] [Article Influence: 122.6] [Reference Citation Analysis (3)] |
| 20. | Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 843] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 21. | Nwokolo CU, Freshwater DA, O'Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Suzuki H, Masaoka T, Hosoda H, Ota T, Minegishi Y, Nomura S, Kangawa K, Ishii H. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Aydin F, Kaklikkaya N, Ozgur O, Cubukcu K, Kilic AO, Tosun I, Erturk M. Distribution of vacA alleles and cagA status of Helicobacter pylori in peptic ulcer disease and non-ulcer dyspepsia. Clin Microbiol Infect. 2004;10:1102-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | el-Omar E, Penman I, Dorrian CA, Ardill JE, McColl KE. Eradicating Helicobacter pylori infection lowers gastrin mediated acid secretion by two thirds in patients with duodenal ulcer. Gut. 1993;34:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 196] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Moss SF, Calam J. Acid secretion and sensitivity to gastrin in patients with duodenal ulcer: effect of eradication of Helicobacter pylori. Gut. 1993;34:888-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 4.3] [Reference Citation Analysis (0)] |