Published online Mar 14, 2007. doi: 10.3748/wjg.v13.i10.1561
Revised: December 15, 2006
Accepted: February 12, 2007
Published online: March 14, 2007
AIM: To evaluate the expression of C-X-C motif chemokine receptor 4 (CXCR4) and its signaling cascades, which were previously identified as a key factor for cancer cell progression and metastasis, in cholangiocarcinoma cell lines.
METHODS: The expression of CXCR4 and its signaling cascades were determined in the cholangiocarcinoma cell lines (RMCCA1 and KKU100) by Western blotting. The invasion assays and the detection of actin polymerization were tested in these cholangiocarcinoma cells treated with CXC chemokine ligand -12 (CXCL12).
RESULTS: Expression of CXCR4 was detected in both cholangiocarcinoma cell lines and activation of CXCR4 with CXCL12 triggered the signaling via the extracellular signal-regulated kinase-1/2 (ERK1/2) and phosphoinositide 3-kinase (PI3K) and induction of cholangiocarcinoma cell invasion, and displayed high levels of actin polymerization. Addition of CXCR4 inhibitor (AMD3100) abrogated CXCL12-induced phosphorylation of MEK1/2 and Akt in these cells. Moreover, treatment with MEK1/2 inhibitor (U0126) or PI3K inhibitor (LY294002) also attenuated the effect of CXCL12-induced cholangiocarcinoma cell invasion.
CONCLUSION: These results indicated that the activation of CXCR4 and its signaling pathways (MEK1/2 and Akt) are essential for CXCL12-induced cholangiocarcinoma cell invasion. This rises Implications on a potential role for the inhibition of CXCR4 or its signal cascades in the treatment of cholangiocarcinoma.
- Citation: Leelawat K, Leelawat S, Narong S, Hongeng S. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol 2007; 13(10): 1561-1568
- URL: https://www.wjgnet.com/1007-9327/full/v13/i10/1561.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i10.1561
Cholangiocarcinoma is a malignant tumor composed of cells resembling those of the biliary tract epithelium[1,2]. The incidence of and mortality rate for cholangiocarcinoma varied considerably in different geographic regions, with the incidence highest in Southeast Asia especially in Thailand[3]. Three-year survival rates of 35% to 50% are achieved in only a few numbers of patients when negative histological margins are attained at the time of surgery[2,4-6]. The causes of lethality of this disease are not only its rapid growth but also the tendency to invade adjacent organs and metastasize[4-6].
At present, a number of molecules implicated in the metastasis processes of cholangiocarcinoma cells have been identified[7-9]. However, there have been no studies exploring the precise mechanisms determining the directional of invasion of cholangiocarcinoma cells into specific organs. In this regard, chemokines are a superfamily of small proteins that bind to G protein-coupled receptors on target cells[10,11]. CXC chemokine ligand-12 (CXCL12)-or stromal cell-derived factor-1 (SDF1) is a member of CXC chemokine family, which was initially cloned from murine bone marrow and characterized as a pre-B-cell growth stimulating factor. CXCL12 exerts effects through its cognate receptor C-X-C motif chemokine receptor 4 (CXCR4), which is the only physiological receptor for CXCL12 and is known to play roles in leukocyte homing as well as metastasis of many kinds of cancer cells[12-15]. A previous study demonstrated that CXCL12 released from fibroblasts induced the increase in migration of cholangiocarcinoma cells[16]. However, the signal transduction pathways following CXCR4 activation and stimulation of cholangiocarcinoma cell invasion have not been delineated.
In this study, we have demonstrated the expression of CXCR4 in human cholangiocarcinoma cell lines. Using in vitro model systems, we demonstrated the activation of CXCR4 by CXCL12 induced phosphorylation of the MEK1/2 and Akt and also enhanced cholangiocarcinoma cells invasion. In addition, administration of AMD3100, a bicyclam noncompetitive antagonist of CXCR4 or inhibition of its signal transduction intermediate molecules (MEK1/2 and PI3K) suppressed the invasiveness of cholangiocarcinoma cells.
HAM’s F12 medium and fetal bovine serum were purchased from Gibco (Grand Island, NY, USA). The recombinant human CXCL12, polyclonal antibodies to human CXCL12 and CXCR4 were purchased from Abcam (Cambridge, MA, USA). Polyclonal antibodies to MEK-1/2 (phosphorylated at Ser217/221 and total), Akt (phosphorylated at Ser473 and total) were purchased from Cell Signaling (Cell Signaling Technology, Beverly, MA, USA). 24-well Biocoat Matrigel invasion chambers (8 μm) were purchased from Becton Dickinson (Becton Dickinson, Franklin Lakes, NJ, USA).
Two human cholangiocarcinoma cell lines; KKU100 derived from Hilar-cholangiocarcinoma patient[17] (kindly provided by Dr. Banchob Sripa, Department of Pathology, Faculty of Medicine, Khon Kaen University) and RMCCA1 derived from Peripheral-cholangiocarcinoma patient[18] were grown in HAM’s F12 medium supple-mented with 100 mL/1L fetal bovine serum at 37°C in a 50 mL/L CO2 humidified atmosphere. For signal transduction experiments with CXCL12, cells were starved overnight in serum-free medium.
For Western blot analysis, 500 000 cells were seeded in a six-well culture plate, followed by treatment with 100 ng/mL of CXCL12. Cells were collected and then Western blot analyses were performed as previously described[18]. Chemiluminescent detection of antibody–antigen complexes revealed the target proteins on X-ray film.
Cells were seeded in 96-well culture plates at a density of 10 000 cells per well followed by the addition of CXCL12 in various concentrations. Then cells were incubated for indicated time before applying the WST-1 cell proliferation assay reagent (Roche Diagnostics, Laval, Quebec) according to the recommendation of the manufacturer. The percentage of proliferation was calculated based on the untreated cells.
The migration of cholangiocarcinoma cells was assayed using chamber with 8-μm pore filters (Transwell, 24-well cell culture, Coster, Boston, MA). 50 000 cholangiocarcinoma cells were added to the upper chamber. Then 0.5 mL serum-free media with 100 ng/mL of CXCL12 was added to the lower chamber. The chambers were incubated for 12 h at 37°C. After incubation, the filters were fixed and stained with hematoxylin and counted in five random high-power fields under a light microscope[19].
The invasion of cholangiocarcinoma cells was assayed in 24-well Biocoat Matrigel invasion chamber (8 μm; Becton Dickinson, Franklin Lakes, NJ). Similar to the migration assays, 50 000 cells were seeded in the upper chamber while the bottom chamber contained with 100 ng/mL of CXCL12.
Cholangiocarcinoma cells were starved by culturing in serum-free medium containing with or without CXCL12 for 24 h before collection of the conditioned medium. The conditioned medium was stored at -80°C and analyzed for MMP9 activity by gelatin zymography.
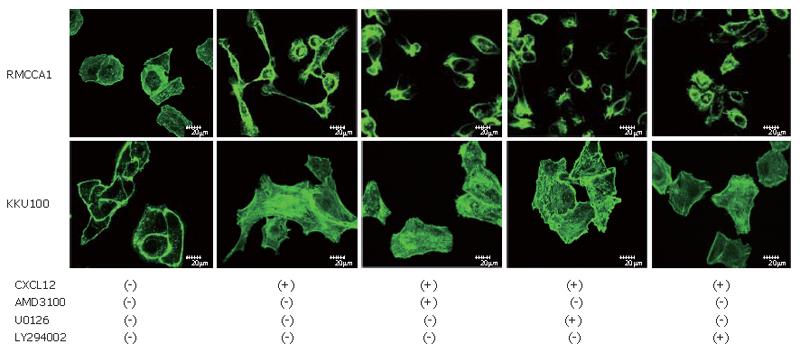
Detection of actin polymerization was performed as described previously[18]. Cholangiocarcinoma cells were treated with AMD3100, U0126, LY294002 or control and incubated for 6 h. Then the cells were incubated in serum-free medium containing with 100 ng/mL of CXCL12 for 4 h. The cells were exposed to Alexa Fluor 488 Phalloidin (Molecular Probes, Eugene, OR) for 30 min and washed with PBS. The cells were examined under a Confocal laser scanning microscope (CLSM), (Olympus SV1000).
The experiments were all performed in triplicate and identical results were obtained. Values were expressed as the mean and SD. The student’s t-test was used for analysis of the cell proliferation and invasion assay. The P value of less than 0.05 was considered significant.
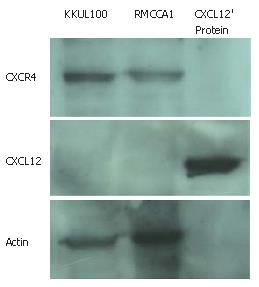
In order to utilize an in vitro system to study the influence of CXCR4 activation, the expression of CXCR4 and CXCL12 in two cholangiocarcinoma cell lines (RMCCA1 and KKU100) needed to be investigated. Western blot analysis demonstrated definite expression of CXCR4 but not CXCL12 in both cholangiocarcinoma cell lines (Figure 1).
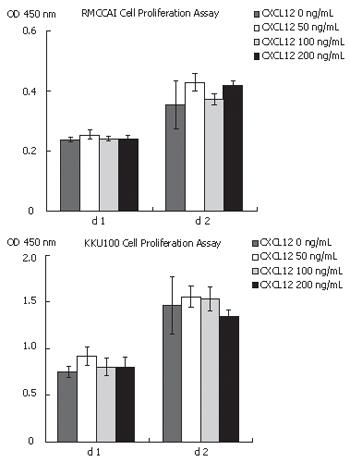
Since the activation of CXCR4 with CXCL12 was known to play an important role in cell proliferation in many kinds of cancer cells, we investigated the role of CXCL12 in cholangiocarcinoma cell proliferation. Cell proliferation assay was performed in RMCCA1 and KKU100 cells treated with CXCL12 at concentrations of 0, 50, 100 and 200 ng/mL. After 48 h of incubation, the results showed that CXCL12 had no effect on cholangiocarcinoma cell proliferation (Figure 2).
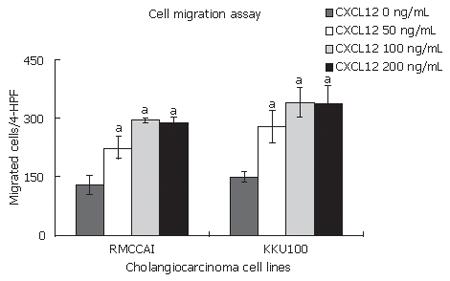
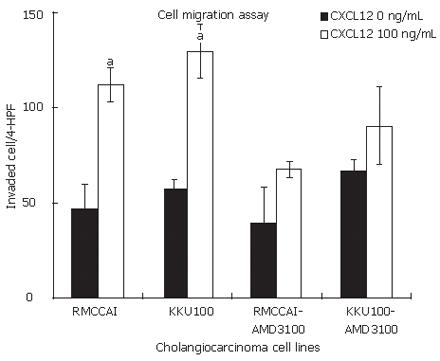
We found that CXCL12 induced the migration of RMCCA1 and KKU100. Their maximum effect was identified at 100 ng/mL of CXCL12 (Figure 3). Therefore, the following cell invasion experiments were performed by using CXCL12 at a concentration of 100 ng/mL. CXCL12 significantly enhanced cholangiocarcinoma cell invasion when compared with untreated cells (Figure 4). To confirm the mechanism by which CXCR4 induced invasion of cholangiocarcinoma cells, RMCCA1 and KKU100 cells were pre-treated with AMD3100, a specific inhigitor for CXCR4, and then treated with CXCL12 for evaluation of cell invasion activity. The invasion induced by CXCL12 was inhibited by AMD3100 in both cholangiocarcinoma cell lines (Figure 4).
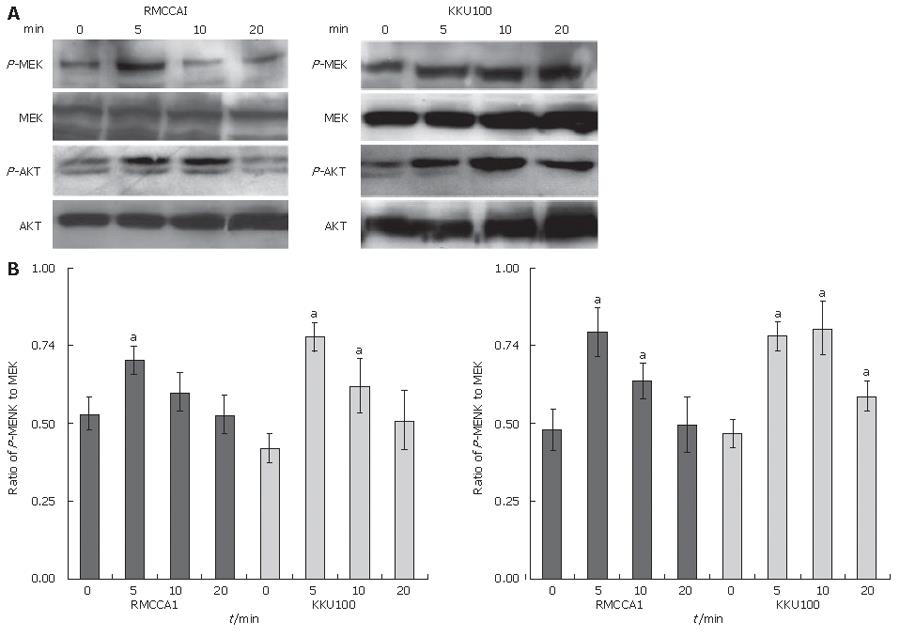
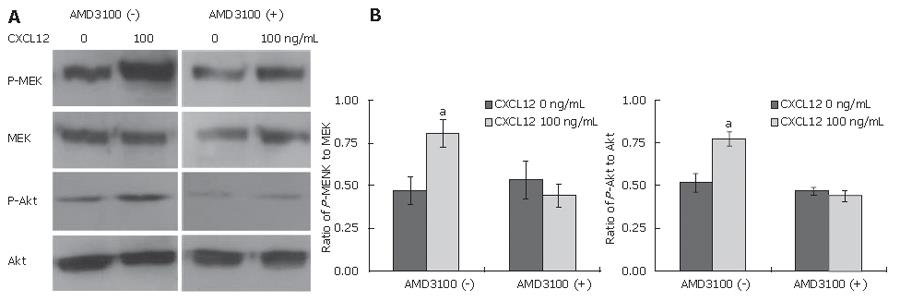
We attempted to evaluate the signaling pathways relevant to the CXCL12 induction of cholangiocarcinoma cell invasion. The phosphorylation of molecules, which were previously demonstrated as CXCR4, mediated signaling molecules, was assayed by Western blot analysis. CXCL12-treated cells demonstrated a higher extent of the phosphorylated MEK1/2 and Akt than untreated cells (Figure 5). To determine whether the activation of CXCR4 induced phosphorylation of these signal transduction molecules, cells were pre-treated with AMD3100. The phosphorylation of MEK1/2, and Akt in AMD3100 pre-treated cells was significantly lower than in untreated cells (Figure 6).
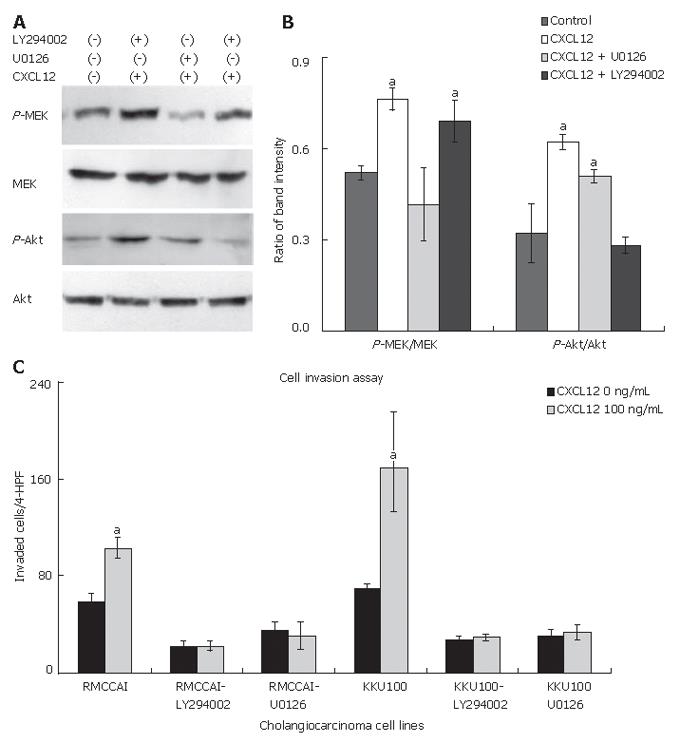
The ability of MEK1/2 inhibitor (U0126) and PI3k inhibitor (LY294002) to decrease the effect of CXCL12-induced phosphorylation of MEK1/2 and Akt was assessed. The MEK1/2 inhibitor (U0126) suppressed CXCL12-induced MEK1/2 phosphorylation and the PI-3K inhibitor (LY294002) suppressed CXCL12-induced Akt phosphorylation (Figure 7A and B). To evaluate the contribution of the MEK1/2 or PI3K pathways to CXCL12-induced cholangiocarcinoma cell invasion, RMCCA1 and KKU100 cells were pre-treated with U0126 or LY294002 and then treated with CXCL12. The invasion induced by CXCL12 was inhibited by U0126 or LY294002 in both cholangiocarcinoma cell lines (Figure 7C). These results strongly suggest that the activation of MEK1/2 and PI3K signaling pathways is essential for CXCL12-induced cholangiocarcinoma cell invasion.
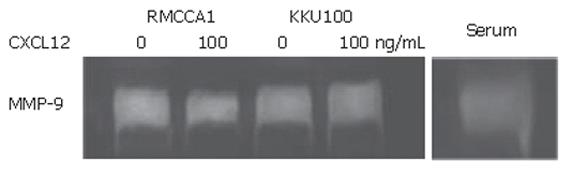
Previous studies have demonstrated that CXCL12 induced MMP-9 activation. Therefore, we investigated the effect of CXCL12 on MMP-9 activation by gelatin zymography from condition medium of cholangiocarcinoma cells. The results showed that prominent constitutive MMP-9 activation was observed in both cholangiocarcinoma cell lines. However, activation of CXCR4 by CXCL12 had no influence on MMP-9 activation (Figure 8).
The ability of cancer cell invasion requires coordinated activation of extracellular matrix degradation and cell motility mechanism. The cell motility was assessed by checking the actin polymerization. Cholangiocarcinoma cells were stained with phalloidin for detection of actin polymerization. Serum-starved cells showed low levels of actin polymerization. After the treatment with 100 ng/mL of CXCL12, cholangiocarcinoma cells displayed high levels of actin polymerization in the periphery of the cells and a distinct pseudopodia formation. Treatment of cholangiocarcinoma cells with AMD3100, U0126 or LY294002 before addition of CXCL12 caused eradication of actin polymerization (Figure 9).
Cholangiocarcinoma is a disease with dismal prognosis characterized by early vascular invasion and metastasis. Therapeutic options for cholangiocarcinoma have been limited due to poor response to chemotherapy and radiation therapy. Surgery is perhaps the only effective treatment for cholangiocarcinoma[2,4]. Previous studies suggested that the most important prognostic factor is a tumor-free surgical margin while other features that were associated with a poor prognosis include factors connected to the extent of disease that is caused by cancer cell invasion, such as bilobar distribution, lymph node involvement, vascular invasion and distant metastases[4,5]. Therefore, an understanding of the mechanism of cholangiocarcinoma cell invasion will be a decisive step towards the develop-ment of targeted tumor-specific therapies.
Chemokines and their receptors are involved in the process of cell migration during inflammation. Recently, studies implicated CXCR4 in chemotaxis, invasiveness and metastasis of tumors, particularly in metastasis of breast cancer, in an organ-specific manner[13,20]. In this present work, we report the results of our studies of CXCR4 and CXCL12 expression in two kinds of human cholangiocarcinoma cell lines; KKU100 derived from the hilar-cholangiocarcinoma patient and RMCCA1 derived from the peripheral-cholangiocarcinoma patient. Both cell lines are expressed CXCR4 but not CXCL12. These findings imply a paracrine effect of CXCR4/CXCL12 rather than an autocrine such in both cholangiocarcinoma cell lines.
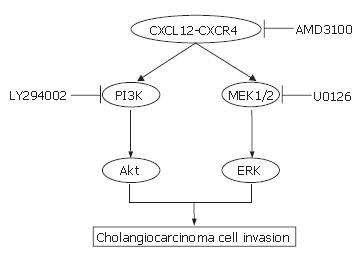
In the present study, the effect of CXCL12 on CXCR4 in two cholangiocarcinoma cell lines was tested in vitro by using cell proliferation, cell migration and cell invasion assays. The findings provided interesting data on the possible molecules of significance involved in promoting cholangiocarcinoma cell invasion. Despite suggestions in previous reports that CXCL12 was a potent stimulator for small cell lung cancer cell proliferation[21], this present study included others arrived to exactly the opposite conclusions[22,23]. In cholangiocarcinoma cell lines, we identified that CXCL12 had no direct effect on cell proliferation. We suggested that these differences might be due to the different culture system or to different target cells. We identified that both cholangiocarcinoma cell lines expressed CXCR4 and stimulation of CXCR4 with CXCL12 promotes cancer cell migration and invasion. Moreover, we also found that KKU100 had a higher invasiveness property than RMCCA1. This result was related with the high expression of CXCR4 in KKU100. Our studies suggested that these events may involve the activation of the ERK1/2 and PI3K. Previous studies have demonstrated that activation of ERK1/2 by G-protein-coupled receptors occurred via the Raf/MEK1/2/ERK1/2 cascade while activated PI3K converted phosphatidylinositol 4, 5 phosphate (PIP2) into phosphatidylinositol 3, 4, 5 phosphate (PIP3), which results in the membrane localization of Akt[24,25]. The latter assertion is based on the finding that inhibition of CXCR4 by AMD3100 suppressed the phosphorylation of MEK1/2 and Akt and also inhibited the invasiveness properties of cholangiocarcinoma cells. Moreover, the addition of MEK1/2 inhibitor (U0126) or PI3K inhibitor (LY294002) also attenuated the effect of CXCL12-induced cholangiocarcinoma cell invasion. To the best of our knowledge, this present study is the first report demonstrating the signal transduction pathways of CXCR4 in cholangiocarcinoma. The targets of AMD3100, U0126 and LY294002 (CXCR4, MEK1/2 and PI3K, respectively) are shown in Figure 10. These results are consistent with the previous studies that demonstrating the activation of ERK and Akt signaling after stimulation of cancer cells with CXCL12[22,26,27].
In cancer cells, high level of actin polymerization is a key for the formation of pseudopodia, which in turn are implicated in the enhancement of cancer cell migration and invasion[28]. Treatment of cholangiocarcinoma cells with CXCL12 resulted in our study in the increase in actin polymerization. In addition, inhibiting CXCR4 with AMD3100 in our study resulted in a dramatic decrease in actin polymerization. Our findings suggest that CXCL12 and CXCR4 play an important role in the invasion as well as the metastasis in cholangiocarcinoma. Previous studies have demonstrated the influence of CXCL12 on MMP-9 secretion[26,27]. In cholangiocarcinoma cell lines, we showed that CXCL12 had no effect on MMP-9 secretion. The mechanisms responsible for MMP-9 activation in cholangiocarcinoma cells remain unclear. We suggested that the mechanism of cholangiocarcinoma cell invasion is not dependent on a single oncogenic pathway but possible complex networks of ligands and their receptors are implicated in cancer invasion such as c-Met/HGF[18], focal adhesion kinase[29] and TNFα/TNF receptor as well[30].
In conclusion, in this present experimental study, we show that the stimulation of CXCL12/CXCR4 plays an important role in cholangiocarcinoma cell invasion by the induction of MEK1/2 and Akt signal transductions and stimulation of actin polymerization. Inhibition of CXCL12/CXCR4 and its pathway may represent one of the potential approaches in cholangiocarcinoma therapy.
The authors wish to thank Miss Yuvadee Siriyasub, Center of Nanoimaging (CNI), Faculty of Science, Mahidol University for her excellent technical support in the Confocal laser scanning microscopy analysis.
S- Editor Liu Y L- Editor Hennenberg M E- Editor Che YB
| 1. | Weinbren K, Mutum SS. Pathological aspects of cholangiocarcinoma. J Pathol. 1983;139:217-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 145] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 848] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 4. | Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Zhang BH, Cheng QB, Luo XJ, Zhang YJ, Jiang XQ, Zhang BH, Yi B, Yu WL, Wu MC. Surgical therapy for hiliar cholangiocarcinoma: analysis of 198 cases. Hepatobiliary Pancreat Dis Int. 2006;5:278-282. [PubMed] |
| 6. | Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004;24:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Jinawath N, Chamgramol Y, Furukawa Y, Obama K, Tsunoda T, Sripa B, Pairojkul C, Nakamura Y. Comparison of gene expression profiles between Opisthorchis viverrini and non-Opisthorchis viverrini associated human intrahepatic cholangiocarcinoma. Hepatology. 2006;44:1025-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Jinawath A, Akiyama Y, Yuasa Y, Pairojkul C. Expression of phosphorylated ERK1/2 and homeodomain protein CDX2 in cholangiocarcinoma. J Cancer Res Clin Oncol. 2006;132:805-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Lai GH, Zhang Z, Shen XN, Ward DJ, Dewitt JL, Holt SE, Rozich RA, Hixson DC, Sirica AE. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129:2047-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Kehrl JH. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol Res. 2006;34:211-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Pease JE, Williams TJ. Chemokines and their receptors in allergic disease. J Allergy Clin Immunol. 2006;118:305-318; quiz 319-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Moore MA. The role of chemoattraction in cancer metastases. Bioessays. 2001;23:674-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3911] [Cited by in RCA: 3966] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 14. | Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, Hedin KE. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Majka M, Ratajczak MZ. Biological role of the CXCR4-SDF-1 axis in normal human hematopoietic cells. Methods Mol Biol. 2006;332:103-114. [PubMed] |
| 16. | Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005;11:3392-3397. [PubMed] |
| 18. | Leelawat K, Leelawat S, Tepaksorn P, Rattanasinganchan P, Leungchaweng A, Tohtong R, Sobhon P. Involvement of c-Met/hepatocyte growth factor pathway in cholangiocarcinoma cell invasion and its therapeutic inhibition with small interfering RNA specific for c-Met. J Surg Res. 2006;136:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Leelawat K, Ohuchida K, Mizumoto K, Mahidol C, Tanaka M. All-trans retinoic acid inhibits the cell proliferation but enhances the cell invasion through up-regulation of c-met in pancreatic cancer cells. Cancer Lett. 2005;224:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Fernandis AZ, Prasad A, Band H, Klösel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, Sattler M, Johnson BE, Salgia R. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62:6304-6311. [PubMed] |
| 22. | Mori T, Doi R, Koizumi M, Toyoda E, Ito D, Kami K, Masui T, Fujimoto K, Tamamura H, Hiramatsu K. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 370] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 23. | Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG, Janowska-Wieczorek A. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100:2597-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 244] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93:E44-E52. [PubMed] |
| 25. | McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D'Assoro AB. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 504] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 26. | Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate. 2006;66:32-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Brand S, Dambacher J, Beigel F, Olszak T, Diebold J, Otte JM, Göke B, Eichhorst ST. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res. 2005;310:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Lambrechts A, Van Troys M, Ampe C. The actin cytoskeleton in normal and pathological cell motility. Int J Biochem Cell Biol. 2004;36:1890-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Mon NN, Hasegawa H, Thant AA, Huang P, Tanimura Y, Senga T, Hamaguchi M. A role for focal adhesion kinase signaling in tumor necrosis factor-alpha-dependent matrix metalloproteinase-9 production in a cholangiocarcinoma cell line, CCKS1. Cancer Res. 2006;66:6778-6784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Tanimura Y, Kokuryo T, Tsunoda N, Yamazaki Y, Oda K, Nimura Y, Naing Mon N, Huang P, Nakanuma Y, Chen MF. Tumor necrosis factor alpha promotes invasiveness of cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett. 2005;219:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |