Published online Mar 14, 2007. doi: 10.3748/wjg.v13.i10.1534
Revised: January 17, 2007
Accepted: February 13, 2007
Published online: March 14, 2007
AIM: To examine whether and how rosiglitazone enhances apoptosis induced by fluorouracil in human colon cancer (HT-29) cells.
METHODS: Human colon cancer HT-29 cells were cultured in vitro and treated with fluorouracil and/or rosiglitazone. Proliferation and growth of HT-29 cells were evaluated by MTT assay and trypan blue exclusion methods, respectively. The apoptosis of HT-29 cells was determined by acridine orange/ethidium bromide staining and flow cytometry using PI fluorescence staining. The expressions of peroxisome proliferator-activated receptor γ (PPARγ), Bcl-2 and Bax in HT-29 cells were analyzed by Western blot.
RESULTS: Although rosiglitazone at the concentration below 30 μmol/L for 72 h exerted almost no inhibitory effect on proliferation and growth of HT-29 cells, it could significantly enhance fluorouracil-induced HT-29 cell proliferation and growth inhibition. Furthermore, 10 μmol/L rosilitazone did not induce apoptosis of HT-29 cells but dramatically enhanced fluorouracil-induced apoptosis of HT-29 cells. However, rosiglitazone did not improve apoptosis induced by fluorouracil in HT-29 cells pretreated with GW9662, a PPARγ antagonist. Meanwhile, the expression of Bax and PPARγ was up-regulated, while the expression of Bcl-2 was down regulated in HT-29 cells treated with rosiglitazone in a time-dependent manner. However, the effect of rosiglitazone on Bcl-2 and Bax was blocked or diminished in the presence of GW9662.
CONCLUSION: Rosiglitazone enhances fluorouracil-induced apoptosis of HT-29 cells by activating PPARγ.
- Citation: Zhang YQ, Tang XQ, Sun L, Dong L, Qin Y, Liu HQ, Xia H, Cao JG. Rosiglitazone enhances fluorouracil-induced apoptosis of HT-29 cells by activating peroxisome proliferator-activated receptor γ. World J Gastroenterol 2007; 13(10): 1534-1540
- URL: https://www.wjgnet.com/1007-9327/full/v13/i10/1534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i10.1534
Colon cancer is a leading cause of cancer-related death in developed countries[1]. Fluorouracil (5-Fu) is one of the most widely used chemotherapeutic drugs in the treatment of advanced colorectal cancer patients[2]. However, the patient response to this single anticancer agent is 10%-30%[2]. Several mechanisms are responsible for resistance of tumor cells to fluorouracil. On the other hand, the dose increments of systemic administration of 5-Fu would generate unacceptable levels of toxicity to the normal cells of bone marrow and gastrointestinal tract[3]. Therefore, many attempts have been made to enhance its therapeutic effectiveness and reduce its toxicity[4,5]. As we known, the common strategies are to develop and validate new chemopreventive and therapeutic approaches to colon cancer by making use of chemosensitizers or combination of drugs.
It was recently reported that rosiglitazone (Ros), a well-established oral antidiabetic agent, can protect against myelotoxicity produced by fluorouracil[6]. Sik Lee also reported that rosiglitazone attenuates cisplatin-induced renal damage[7].
Rosiglitazone is a member of the thiaolidinediones (TZDs) and a synthetic ligand of the peroxisome proliferator-activated receptor γ (PPARγ)[8]. Members of thiazolidenediones such as troglitazone and ciglitazone exhibit anti-tumor effects on various types of cancer cells, including colon cancer cells expressing high levels of PPARγ[9]. However, low bioavailability of rosiglitazone[10] limits its application in clinical cancer therapy. We thus investigated the effect of rosiglitazone in combination with fluorouracil on human colon cancer cells.
PPARγ has been implicated in metabolic diseases[11,12] and is associated with cell proliferation, differentiation and apoptosis[13]. However, the role of PPARγ in fluorouracil-induced apoptosis of HT-29 cells is unknown.
It was reported that ciglitizone induces significant apoptosis of HT-29 cells and reduces Bcl-2 expression by activating PPARγ[14]. On the other hand, Bcl-2 exerts its functions by heterodimerizing with Bax, a protein that accelerates apoptosis. Deficient expression of Bax is also associated with apoptosis resistance. We thus analyzed the effect of rosiglitazone on the expression of Bax and Bcl-2 in HT-29 cells to understand the underlying mechanisms of 5-Fu-induced apoptosis.
In the present study, we investigated whether and how rosiglitazone enhances fluorouracil-induced apoptosis of HT-29 cells. The results demonstrate that rosiglitazone at low concentration has no inhibitory effect on HT-29 cell growth and proliferation, but enhances apoptosis of HT-29 cells induced by fluorouracil. The mechanism of rosiglitazone underlying the improvement of fluorouracil-induced apoptosis may be associated with Bax and Bcl-2 depending on PPARγ.
Propidium iodide (PI), acridine orange (AO), ethidium bromide (EB) were purchased from Sigma Chemical Company (St. Louis, MO, USA). RPMI-1640 medium and newborn calf serum were supplied by Giboco BRL (Grand Island, NY, USA). Methyl thiazolyl tetrazolium (MTT) and dimethyl sulfoxide (DMSO) were bought from Sigma. Polyclonal antibodies against PPARγ, Bcl-2 and Bax were purchased from Santa Cruz Biotech Co. Horseradish peroxidase-conjugated goat antimouse IgG and goat antirabbit IgG were purchased from Santa Cruz biotechnology, Inc.
Human colon cancer HT-29 cells were obtained from the China Center for Type Culture Collection (Wuhan, China). HT-29 cells were cultured in RPMI-1640 medium supplemented with 10% newborn calf serum, 80 U/mL penicillin and 100 U/mL streptomycin in humidified atmosphere (90% relative humidity) with 5% CO2 at 37°C. The culture media were changed every two days.
HT-29 cells were plated onto 96-well plates at approxi-mately 1.0 × 104 cells per well and incubated for 12 h. The cells were treated with rosiglitazone or fluorouracil or both at various concentrations for 72 h. Then 20 μL of 5 g/mL MTT in phosphate-buffered saline (PBS) was added. The plates were incubated for 4 h and formosan was dissolved in 100 μL DMSO. The absorbance at 570 nm was recorded using an enzyme-linked immunosorbent assay reader. The proliferation inhibition rate (IR) was calculated according to the following formula: IR% = [1-absorbance of drug treatment group/absorbance of vehicle control group] × 100%[15]. The IR was analyzed using Calcusyn program to determine the IC50 of each drug. The combination index (CI)-isobologram by Chou and Talalay[16] was used to analyze the drug combination: CI = IC50(AB)/(IC50(A) + IC50(B)) (A, B represent different drugs).
CI > 1, CI = 1 and CI < 1 indicate antagonism, additive effect, or synergism, respectively.
HT-29 cells were plated onto 24-well plates at approxi-mately 1.0 × 104 cells per well and incubated for 12 h. The cells were treated with rosiglitazone or fluorouracil or both at various concentrations. On d 1, 2, 3, 4 and 5, the cells were harvested by trypsinization and counted under microscope after trypan blue staining. Three independent experiments were carried out based on the following formula: cell viability% = number of cells in drug treatment group/ number of cells in control group × 100%[17]. Population doubling time was calculated as follows: TD = t㏒2/㏒Nt-㏒N0, where TD is population doubling time, t is cell culture time, N0 and Nt are the number of cells at initiation and t time, respectively).
Cells were treated with rosiglitazone or fluorouracil or both for 72 h, then harvested with 0.25% trypsin and resuspended in PBS. After staining for 10 min with 10 μL of 100 mg/mL acridine orange/ethidium bromide (AO/EB) mixture, cells were visualized immediately under a fluorescence microscope (TE2000-S, Nikon, USA)[18].
Cells were treated with rosiglitazone or fluorouracil or both for 72 h, then harvested with 0.25% trypsin and washed with PBS. Cells at a density of 1 × 106 were fixed in 70% ice-cold ethos/PBS and stored at 4°C overnight, then washed with PBS and incubated in PI solution (69 mom PI, 388 mom sodium citrate, 100 go/mL Raze A) for 15 min at 37°C. Cells were immediately analyzed with a FAC scan flow cytometer (Becton Dichinson, San Jose, USA)[17].
Cells were lysed in a lysis buffer containing 0.1 mol/L Nacl, 0.01 mol/L Tris-Cl, 0.001 mol/L EDTA, 1 μmol/L aprotinin, and 100 μmol/L phenylmethylsulfonyl fluoride (PMSF) at 4°C with sonication. The lysates were centrifuged at 15 000 ×g for 15 min and the concentration of protein was determined with a bicinchoninic acid protein assay kit (Pierce Chemicals), using bovine serum albumin as a standard. Loading buffer (42 mmol/L Tris-Cl, 10% glycerol, 2.3% SDS, 5% 2-mercaptoethanol and 0.02% bromophenol blue) was then added to each lysate, which was subsequently boiled for 5 min and electrophoresed on a SDS-polyacrylamide gel. Proteins were transferred onto a polyvinylidene difluoride membrane (PVDF), and incubated separately with antibodies against PPARγ, Bcl-2, Bax and β-actin, and then labled with horseradish peroxidase-conjugated secondary antibodies. The reactions were visualized using an enhanced chemiluminescence reagent (Santa Cruz). The results were approved by repeating the reaction 3 times using different samples[19].
Data were expressed as mean ± SD. ANOVA was used to assess the statistical significance of differences. P < 0.05 was considered statistically significant.
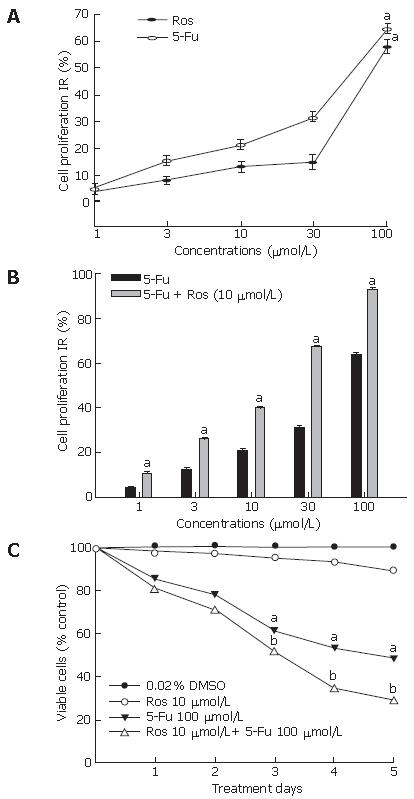
To examine the effect of rosiglitazone on fluorouracil-induced proliferation inhibition of HT-29 cells, the proliferation inhibition rate of HT-29 cells treated with fluorouracil in the presence or absence of rosiglitazone was calculated by MTT method. Rosiglitazone exerted almost no inhibitory effect at the concentration below 30 μmol/L (IR < 20%) for 72 h on HT-29 cells. The IR value for fluorouracil at 30 μmol/L and 100 μmol/L was 30.20% and 64.9%, respectively (Figure 1A). Since the IR value for rosiglitazone at 10 μmol/L was 12.01%, we co-administered 10 μmol/L rosiglitazone with 3, 10, 30, 100 μmol/L of fluorouracil respectively to HT-29 cells. As shown in Figure 1B, fluorouracil inhibited proliferation of HT-29 cells in a dose-dependent manner and rosiglitazone significantly enhanced the proliferation inhibition of HT-29 cells induced by fluorouracil (P < 0.05).
The IC50 of rosiglitazone, fluorouracil or both was 140.4 ± 21.23 μmol/L, 56.9 ± 6.21 μmol/L, 10.5 ± 0.14 μmol/L respectively and the CI value for rosiglitazone and fluorouracil was 0.257, indicating the synergistic effect of combined drugs.
Trypan blue exclusion assay showed that rosiglitazone potently enhanced the susceptibility of HT-29 cells to fluorouracil. Although 10 μmol/L rosiglitazone was not cytotoxic to HT-29 cells, it could dramatically enhance growth inhibition of HT-29 cells stimulated by 100 μmol/L fluorouracil (Figure 1C). When treated with 100 μmol/L 5-Fu, the doubling time of HT-29 cells was 2.5 d, whereas it was 3.4 d in the presence of 10 μmol/L rosiglitazone.
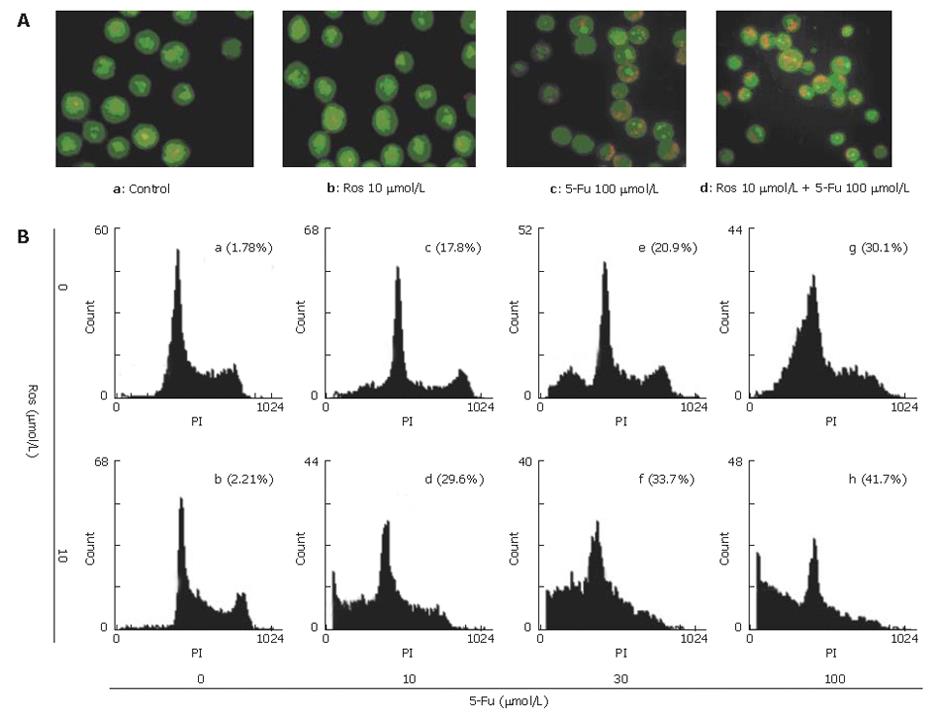
Apoptotic cells were detected by morphological observation using AO/EB staining. As shown in Figure 2A, the normal cells (Figure 2A.a) and cells treated with 10 μmol/L rosiglitazone (Figure 2A.b) exhibited uniformly dispersed chromatin and intact cell membrane. Typical morphological changes were found in apoptotic HT-29 cells exposed to 100 μmol/L fluorouracil for 72 h, including apoptotic nuclear condensation (Figure 2A.c). However, the number of cells with nuclear condensation was significantly increased in cells cotreated with 100 μmol/L fluorouracil and 10 μmol/L rosiglitazone for 72 h (Figure 2A.d), revealing that rosiglita-zone could enhance fluorouracil-induced apoptosis.
To quantify and assess the apoptotic rate of HT-29 cells induced by rosiglitazone in combination with fluorouracil, the proportion of cells that had a DNA content of less than 2N was analyzed by FCM using PI staining (Figure 2B). The apoptosis rate for HT-29 cells treated with 10 μmol/L rosiglitazone for 72 h was 2.1% ± 0.26, which was similar to that for the untreated control group (1.8% ± 0.21). In the presence of 10 μmol/L rosiglitazone, the apoptotic rate for HT-29 cells induced by 10, 30, 100 μmol/L fluorouracil for 72 h was increased from 20.7% ± 0.46%, 23.7% ± 0.43%, and 30.3% ± 0.97 to 28.1% ± 0.70%, 32.7% ± 0.45%, and 40.3% ± 0.73% respectively, indicating that rosiglitazone dramatically promoted apoptosis of HT-29 cells induced by fluorouracil.
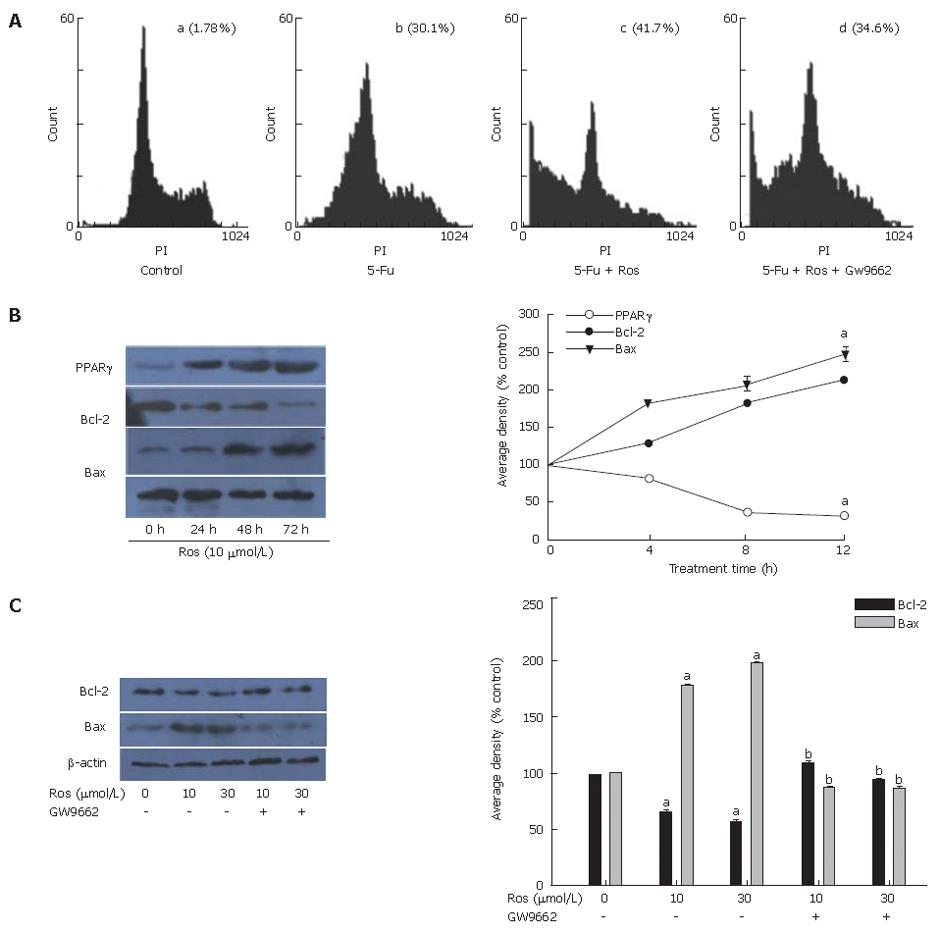
To confirm that rosiglitazone enhances fluorouracil-induced apoptosis of HT-29 cells depending on PPARγ, the effect of GW9662 on fluorouracil–induced apoptosis induced by rosigliatzone was investigated. As shown in Figure 3A , the apoptosis rate of HT-29 cells pretreated with GW9662 30 min before exposed to rosiglitazone and 5-Fu was 33.1% ± 0.81%, lower than that of HT-29 cells not pretreated with GW9662 (40.3% ± 0.73%).
As shown in Figure 3B, the expression of PPARγ and Bax increased in a time-dependent manner, while the expression of Bcl-2 decreased in a time-dependent manner in HT-29 cells treated with 10 μmol/L rosiglitazone for 0, 4, 8, 12 h, respectively.
To confirm the relationship between the expressions of Bcl-2/Bax and PPARγ in HT-29 cells induced by rosiglitazone, HT-29 cells were pretreated with GW9662, a PPARγ antagonist, 30 min before treatment with 10 μmol/L or 30 μmol/L rosiglitazone for 12 h. We found that the expression of Bcl-2 and Bax in HT-29 cells induced by rosiglitazone was blocked by GW9662 (Figure 3C).
A previous study suggested that rosiglitazone inhibits proliferation of the human adrenocortical cancer cell line H295R in a dose-dependent manner with the maximal effect (about 50% inhibition) obtained at 20 μmol/L[20]. Another study also demonstrated that rosiglitazone only at high concentration (> 10 μmol/L) inhibits growth and viability of cancer cells[21]. However, the plasma concentration of rosiglitazone in typical diabetes patients is 1.67 μmol/L[14]. Thus rosiglitazone should not be used as a single anticancer agent.
In this study, rosiglitazone at a low concentration (< 30 μmol/L) did not inhibit HT-29 cell growth in vitro. Importantly 10 μmol/L rosiglitazone promoted fluorouracil-induced proliferation and growth suppression of HT-29 cells. The mechanism may be associated with the low concentration of rosiglitazone promoting fluorouracil-induced apoptosis. When a combination of 10 μmol/L rosiglitazone with various concentrations of 5-Fu was used, the apoptotic rate of HT-29 cells improved compared with 5-Fu alone.
Although rosiglitazone is the most potent and selective synthetic ligand of PPARγ, it suppresses cancer cell growth through PPARγ-dependent and independent[22] signal path ways, because different cellular models may be, at least in part, responsible for the discrepancies. In the present study, rosiglitazone increased PPARγ expression in a time-dependent manner. More importantly, the effect of fluorouracil-induced apoptosis induced by rosiglitazone was blocked by GW9662, suggesting that fluorouracil-induced apoptosis induced by rosiglitazone depends on PPARγ.
Fluorouracil has been known to cause cell injury by inhibiting thymidylate synthesis or by incorporating itself into DNA or RNA[23]. High level expression of thymidylate increases the activity of deoxyuridine triphosphatase[23], methylation of the MLH1 gene, and over expression of Bcl-2, Bcl-XL[24,25]. It was reported that Mcl-1 proteins lead to resistance to 5-Fu[26], suggesting that multiple factors contribute to 5-Fu resistance. It was reported that that ovarian tumors over-expressing Bcl-2 may not respond well to E1A gene therapy, but treatment with a combination of E1A and Bcl-2-ASO may overcome it[27]. Zhu et al[28] found that colon cancer cells resistant to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) can be re-sensitized by a combination therapy of TRAIL and 5-Fu. In our study, rosiglitazone decreased Bcl-2 expression in HT-29 cells, suggesting that fluorouracil-induced apoptosis may reduce HT-29 cell resistance by down regulating Bcl-2 in a time- and dose- dependent manner.
Bcl-2 family can positively and negatively regulate apoptosis[29]. Bcl-2 and Bax are two members of the Bcl-2 family, and play a different role in programmed cell death[30]. When Bax is over-expressed in cells, apoptosis in response to death signals is accelerated, leading to its designation as a death agonist[31]. When Bcl-2 is over-expressed it heterodimerized with Bax and death is repressed[31]. Therefore, the ratio of Bcl-2 to Bax is important in determining susceptibility to apoptosis[31]. In our study, rosiglitazone increased Bax expression in HT-29 cells in a time- and dose- dependent manner, suggesting that rosiglitazone-induced apoptosis may also reduce HT-29 cell resistance by up-regulating Bax expression.
On the other hand, the effect of rosiglitazone on decreasing Bcl-2 level and increasing Bax level in HT-29 cells was blocked by GW9662, suggesting that the enhancing effect of rosiglitazone on apoptosis of HT-29 cells is associated with decreasing Bcl-2/Bax expression by activating PPARγ.
In conclusion, a combination of rosiglitazone and fluorouracil induces strong inhibition of HT-29 cell proliferation and growth. However, the in vivo effect needs further study.
S- Editor Liu Y L- Editor Wang XL E- Editor Chin GJ
| 1. | Austin RP, Barton P, Cockroft SL, Wenlock MC, Riley RJ. The influence of nonspecific microsomal binding on apparent intrinsic clearance, and its prediction from physicochemical properties. Drug Metab Dispos. 2002;30:1497-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 2. | Shin YK, Yoo BC, Chang HJ, Jeon E, Hong SH, Jung MS, Lim SJ, Park JG. Down-regulation of mitochondrial F1F0-ATP synthase in human colon cancer cells with induced 5-fluorouracil resistance. Cancer Res. 2005;65:3162-3170. [PubMed] |
| 3. | Akbulut H, Tang Y, Maynard J, Zhang L, Pizzorno G, Deisseroth A. Vector targeting makes 5-fluorouracil chemotherapy less toxic and more effective in animal models of epithelial neoplasms. Clin Cancer Res. 2004;10:7738-7746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Meta-analysis of randomized trials testing the biochemical modulation of fluorouracil by methotrexate in metastatic colorectal cancer. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1994;12:960-969. [PubMed] |
| 5. | Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, O'Connell M, Sargent P, Piedbois P. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766-3775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Mikhail NE, Cope D. Initiation of insulin in patients with type 2 diabetes failing oral therapy: response to Raskin and Janka. Diabetes Care. 2005;28:1537-1538; author reply 1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Lee S, Kim W, Moon SO, Sung MJ, Kim DH, Kang KP, Jang YB, Lee JE, Jang KY, Park SK. Rosiglitazone ameliorates cisplatin-induced renal injury in mice. Nephrol Dial Transplant. 2006;21:2096-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Strowig SM, Raskin P. The effect of rosiglitazone on overweight subjects with type 1 diabetes. Diabetes Care. 2005;28:1562-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9:1-9. [PubMed] |
| 10. | Cox PJ, Ryan DA, Hollis FJ, Harris AM, Miller AK, Vousden M, Cowley H. Absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. Drug Metab Dispos. 2000;28:772-780. [PubMed] |
| 11. | Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5' flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992;11:433-439. [PubMed] |
| 12. | Fruchart JC, Duriez P, Staels B. Peroxisome proliferator-activated receptor-alpha activators regulate genes governing lipoprotein metabolism, vascular inflammation and atherosclerosis. Curr Opin Lipidol. 1999;10:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 319] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573-25580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 687] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 14. | Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70:2631-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Cao JG, Tang XQ, Shi SH. Multidrug resistance reversal in human gastric carcinoma cells by neferine. World J Gastroenterol. 2004;10:3062-3064. [PubMed] |
| 16. | Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5258] [Cited by in RCA: 5692] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 17. | Hashimoto Y, Shimada Y, Itami A, Ito T, Kawamura J, Kawabe A, Kaganoi J, Maeda M, Watanabe G, Imamura M. Growth inhibition through activation of peroxisome proliferator-activated receptor gamma in human oesophageal squamous cell carcinoma. Eur J Cancer. 2003;39:2239-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Tang XQ, Bi H, Feng JQ, Cao JG. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol Sin. 2005;26:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Li MY, Deng H, Zhao JM, Dai D, Tan XY. PPARgamma pathway activation results in apoptosis and COX-2 inhibition in HepG2 cells. World J Gastroenterol. 2003;9:1220-1226. [PubMed] |
| 20. | Ferruzzi P, Ceni E, Tarocchi M, Grappone C, Milani S, Galli A, Fiorelli G, Serio M, Mannelli M. Thiazolidinediones inhibit growth and invasiveness of the human adrenocortical cancer cell line H295R. J Clin Endocrinol Metab. 2005;90:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Valentiner U, Carlsson M, Erttmann R, Hildebrandt H, Schumacher U. Ligands for the peroxisome proliferator-activated receptor-gamma have inhibitory effects on growth of human neuroblastoma cells in vitro. Toxicology. 2005;213:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Shiau CW, Yang CC, Kulp SK, Chen KF, Chen CS, Huang JW, Chen CS. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer Res. 2005;65:1561-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Okabe H, Tsujimoto H, Fukushima M. The correlation between thymidylate synthase expression and cytotoxicity of 5-fluorouracil in human cancer cell lines: study using polyclonal antibody against recombinant human thymidylate synthase. Gan To Kagaku Ryoho. 1997;24:705-712. [PubMed] |
| 24. | Violette S, Poulain L, Dussaulx E, Pepin D, Faussat AM, Chambaz J, Lacorte JM, Staedel C, Lesuffleur T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int J Cancer. 2002;98:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Liu R, Page C, Beidler DR, Wicha MS, Núñez G. Overexpression of Bcl-x(L) promotes chemotherapy resistance of mammary tumors in a syngeneic mouse model. Am J Pathol. 1999;155:1861-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Bartholomeusz C, Itamochi H, Yuan LX, Esteva FJ, Wood CG, Terakawa N, Hung MC, Ueno NT. Bcl-2 antisense oligonucleotide overcomes resistance to E1A gene therapy in a low HER2-expressing ovarian cancer xenograft model. Cancer Res. 2005;65:8406-8413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Zhu H, Zhang L, Huang X, Davis JJ, Jacob DA, Teraishi F, Chiao P, Fang B. Overcoming acquired resistance to TRAIL by chemotherapeutic agents and calpain inhibitor I through distinct mechanisms. Mol Ther. 2004;9:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3914] [Cited by in RCA: 3936] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 30. | Kirkin V, Joos S, Zörnig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 407] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 31. | Sultana H, Kigawa J, Kanamori Y, Itamochi H, Oishi T, Sato S, Kamazawa S, Ohwada M, Suzuki M, Terakawa N. Chemosensitivity and p53-Bax pathway-mediated apoptosis in patients with uterine cervical cancer. Ann Oncol. 2003;14:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |