Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7508
Revised: October 26, 2006
Accepted: November 3, 2006
Published online: December 14, 2006
AIM: To explore the effects of ultrasound exposure combined with microbubble contrast agent (SonoVue) on the permeability of the cellular membrane and on the expression of plasmid DNA encoding enhanced green fluorescent protein (pEGFP) transfer into human umbilical vein endothelial cells (HUVECs).
METHODS: HUVECs with fluorescein isothiocyanate-dextran (FD500) and HUVECs with pEGFP were exposed to continuous wave (1.9 MHz, 80.0 mW/cm2) for 5 min, with or without a SonoVue. The percentage of FD500 taken by the HUVECs and the transient expression rate of pEGFP in the HUVECs were examined by fluorescence microscopy and flow cytometry, respectively.
RESULTS: The percentage of FD500-positive HUVECs in the group of ultrasound exposure combined with SonoVue was significantly higher than that of the group of ultrasound exposure alone (24.0% ± 5.5% vs 66.6% ± 4.1%, P < 0.001). Compared with the group of ultrasound exposure alone, the transfection expression rate of pEGFP in HUVECs was markedly increased with the addition of SonoVue (16.1% ± 1.9% vs 1.5% ± 0.2%, P < 0.001). No statistical significant difference was observed in the HUVECs survival rates between the ultrasound group with and without the addition of SonoVue (94.1% ± 2.3% vs 91.1% ± 4.1%).
CONCLUSION: The cell membrane permeability of HUVECs and the transfection efficiency of pEGFP into HUVECs exposed to ultrasound are significantly increased after addition of an ultrasound contrast agent without obvious damage to the survival of HUVECs. This non-invasive gene transfer method may be a useful tool for clinical gene therapy of hepatic tumors.
- Citation: Nie F, Xu HX, Tang Q, Lu MD. Microbubble-enhanced ultrasound exposure improves gene transfer in vascular endothelial cells. World J Gastroenterol 2006; 12(46): 7508-7513
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7508.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7508
Tumor angiogenesis plays a critical role in the development and progression of hepatocellular carcinoma (HCC)[1]. The emerging success of anti-angiogenic therapy for other cancers, such as colorectal cancer[2,3] and lung cancer[4], suggests that it may be useful for HCC because of its hyper-vascularity. To the best of our knowledge, the delivery method targeting the vascular endothelial cells of HCC has not been used in gene therapy as yet. As an effective strategy, gene therapy is largely dependent on the development of a vector or vehicle that can selectively and efficiently deliver a gene to cells or target tissues with minimal toxicity. There has been a great deal of attention paid to the development of different gene carriers to more effectively deliver DNA[5]. In addition to naked DNA, both viral (e.g., adenovirus) and non-viral (e.g., liposome) vectors have been used[6,7]. DNA may also be incorporated into nanospheres with biopolymers, such as chitosan and gelatin[8]. Ultrasound exposure has been proven to permeabilize plasma membranes[9] and reduce the thickness of the unstirred layer at the cell surface[10] because of the remarkable ability of ultrasound to produce cavitation activity[11]. Cavitation is the interaction between an ultrasonic field in a liquid and gaseous inclusion (i.e., microbubbles) within the insonated medium, which disrupts the structure of the carrier vesicle and releases the drug and also makes the cell membranes and capillaries more permeable to drugs. Cavitating gas bodies, such as microbubbles, are the mediators through which the energy of relatively non-interactive pressure waves is concentrated to produce forces that permeabilize cell membranes and disrupt the vesicles that carry the gene[12,13]. SonoVue, as a microbubble contrast agent, associated with the gene on the surface, under ultrasound exposure , can lower the threshold for cavitation by acting as a cavitation nucleus and boosts the cavitation which alters cell membrane permeability without significantly affecting cell viability in biological tissue[10], which can be used to enhance gene transfer even further. In this study, we attempted to evaluate the effect of microbubble-enhanced ultrasound exposure in improving the transfection of enhanced pEGFP into HUVECs. The aims of the study were to elucidate the mechanism of microbubble-enhanced transfection and to construct a novel gene therapy that might be used to treat HCC.
Primary human umbilical vein endothelial cells (HUVECs), separated with 0.2% collagenase (Sigma, St. Louis, MO, USA) from newborn aseptic umbilical cords after abdominal delivery from the healthy pregnant women who had normal routine urinalysis and blood tests (women with any complications of pregnancy or viral infections were excluded), were maintained in Human Endothelial-SFM (Gibco, California, USA) supplemented with 100 mL/L fetal bovine serum (Gibco), 2 ng/mL vascular endothelial cell growth factor (Sigma), 90 μg/mL heparin (Sigma), 100 U/mL penicillin and 50 μg/mL streptomycin. HUVECs were cultured in a 50 ml/L CO2 incubator at 37°C[14], and the cellular viability was determined by counting the cells stained with trypan blue (Sangon Biological Engineering technology, Shanghai, China). The HUVECs were identified by detecting rabbit anti-human von Willebrand factor (Boshide Company, Wuhan, China), as well as by performing the acetylated low-density lipoprotein color reagent test (DiI-Ac-LDL) (Molecular Probes, Novato, Canada, Europe BV). Further experiments were performed only when the purity of the HUVECs was above 95%.
The microbubble-based ultrasound contrast agent SonoVueTM (Bracco SpA, Milan, Italy) was used in this study, which consists of 59 mg of sulfur hexafluoride gas (SF6) and 25 mg of a freeze-dried white powder. After adding 5 mL of normal saline into the vial and then shaking for several seconds, phospholipid-stabilized microbubbles filled with sulfur hexafluoride with a diameter of less than 8 μm (mean, 2.5 μm) were generated[15,16].
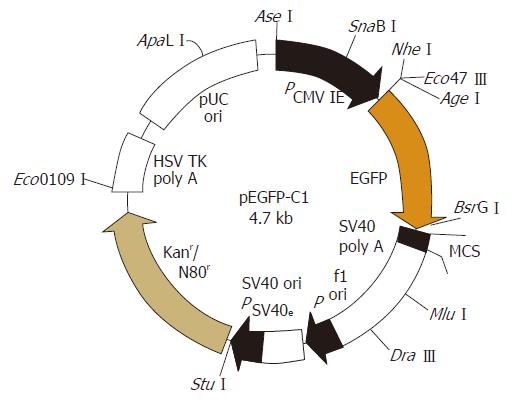
The EGFP plasmid DNA (donated by Doctor Zhangge, Laboratory of Molecular Medicine, Sun Yat-Sen University, China) was prepared with a special reagent (E.ZN.A Plasmid Miniprep Kit II, Omega Bio-tek Company, Doraville, GA, USA). The purity of extract EGFP plasmid DNA was more than adequate given that the value of 260 was 1.8 as measured on ultraviolet spectrophotometry (DU800, BeckMan, Miami, USA). EGFP plasmid DNA was identified by enzyme digestive (SalIor XhoI) and subsequent electrophoresis. The map of pEGFP was analyzed (Figure 1), two restriction sites SalIand XhoIwere included which proved that the obtained plasmid was EGFP.
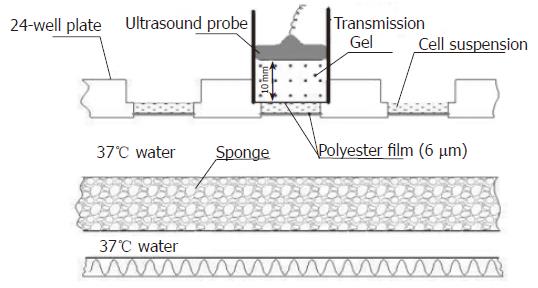
HUVECs were counted and resuspended in 24-well plates at a density of 1.5 × 105 cells/well. Fifty microliters of FD500 (25 mg/mL, Sigma, St. Louis, MO, USA) was added to the 24-well plates. The 24-well plates were randomly assigned into four groups, each consisting of six samples: group A (control group), sham-exposure to ultrasound without addition of SonoVue; group B, sham-exposure to ultrasound with the addition of SonoVue (600 μg/well); group C, exposure to ultrasound without addition of SonoVue; and group D, exposure to ultrasound with the addition of SonoVue (600 μg/well). Ultrasound was delivered using an HP-Viewpoint 2500 scanner (Hewlett-Packard Company, NY, USA). An E94K213 transcranial vector transducer (12 mm in diameter) with transmission gel (thickness, 10 mm) was placed on polyester film (thickness, 6 μm) that covered the 24-well plate, and the cells were exposed to 1.9 MHz continuous ultrasound at an 80.0 mW/cm2 output intensity for 5 min (Figure 2). The cells were incubated for another 4 h. Subsequently, a flow cytometer (Elite, Beckman-Coulter, Miami, USA) was used to detect the percentage of FD500-positive cells in 5 wells from each group (10 000 cells in each well were detected). The other wells from each group were observed for fluorescent staining of HUVECs under the fluorescent microscope (Leica, Danaher Corporation, Wetzlar, Germany).
HUVECs, at a density of 1.5 × 105 cells/well, were resuspended in 24-well plates, and 15.0 μg of pEGFP (280 μg/mL) was added to the 24-well plates. The total amount in each well was approximately 300 μL. The mixture of the HUVECs and pEGFP was incubated for 20 min at 37°C. The 24-well plates were divided into 4 groups, each consisting of 6 samples: group A, no ultrasound exposure and no SonoVue; group B, SonoVue (600 μg/well) only; group C, ultrasound exposure only; and group D, both SonoVue (600 μg/well) and ultrasound exposure. The cells were exposed to 1.9-MHz of continuous waves at an 80.0 mW/cm2 output intensity for 5 min. The cell viability was tested immediately with trypan blue after the exposure was completed. The cell suspensions were harvested from the wells, separated by centrifugation, and resuspended in medium. After 48-h culture, the pEGFP transfection efficiency was detected with fluorescent microscopy and flow cytometry.
Data were expressed as mean ± SD. ANOVA was used to analyze the differences among the different groups. P < 0.05 was considered statistically significant.
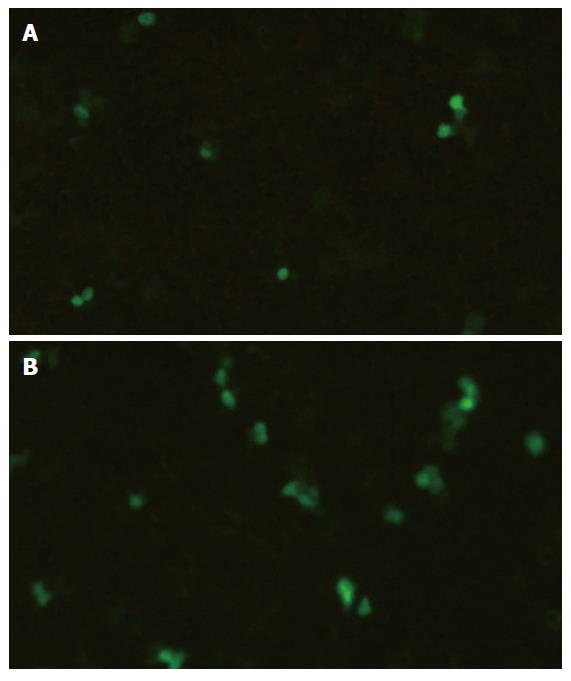
Samples were evaluated with fluorescent microscopy and flow cytometry. Faint fluorescent staining in the cytoplasm was observed in few HUVECs in group A (control group) and group B (stimulated by SonoVue only). A few dispersed fluorescent-stained HUVECs were observed in group C (stimulated by ultrasound only), whereas an increased number and high concentration of fluorescent-stained HUVECs were detected in group D (stimulated by both ultrasound and SonoVue) (Figure 3). The percentages of FD500-positive HUVECs detected by flow cytometry are presented in Table 1.
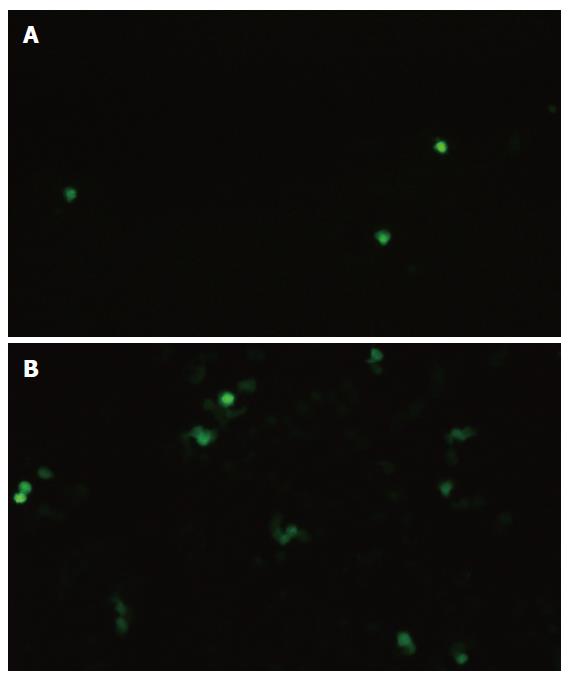
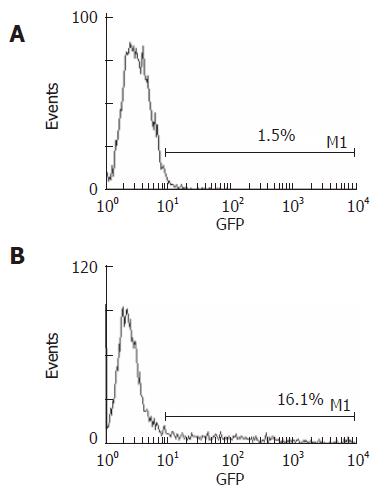
In group D (stimulated by both SonoVue and ultrasound exposure), fluorescent microscopy revealed that the HUVECs transfected by the EGFP gene had an obviously green fluorescence in the cytoplasm, while the cells in other groups had almost no coloration (Figure 4). Flow cytometry showed that HUVECs with ultrasound exposure only barely expressed EGFP, whereas a little more than 16% of HUVECs with both ultrasound exposure and SonoVue expressed EGFP (Figure 5).
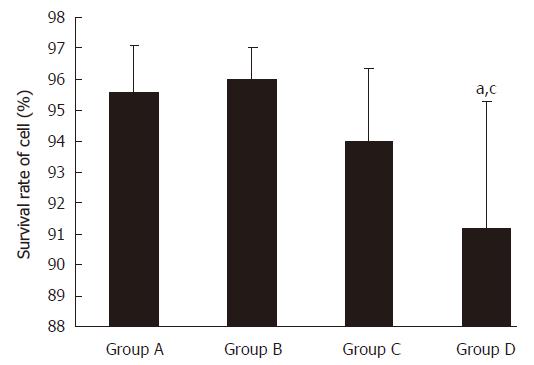
The percentage of surviving cells was examined by trypan test after ultrasound exposure. The cell survival rates were 95.6% ± 1.4% in the control group, 96.0% ± 0.9% in group B, 94.1% ± 2.3% in group C, and 91.1 %± 4.1% in group D (Figure 6).
Gene therapy has made significant progress in the therapy of liver neoplasms[17]. Hepatocellular carcinoma (HCC) is a typical malignancy characterized by neovascularization. Recent studies have shown that the angiogenic activity in HCC is correlated with metastasis and tumor recurrence after resection[18,19]. Angiogenesis provides a target for therapeutic approaches to HCC and may show clinical potential.
The success of gene therapy is largely dependent on the development of the gene delivery vector which is safe, easy to apply and give efficient transgene expression. DNA molecules are large, negatively charged molecules and have major difficulties in entering the cell or cell nucleus. DNA becomes rapidly degraded by extra- and intra-cellular nucleases[20]. Therefore, suitable DNA delivery systems are under development. Viral vectors have been developed as highly efficient carriers for gene delivery to a variety of tissues; the efficiency of these vectors is due to the presence of viral proteins that interact with cell surface receptors. However, viral gene carriers have some major disadvantages: they often provoke an immune response and severe inflammation reactions. Additionally, the risk for insertional mutagenesis and the size
Limitations of the DNA that they can accommodate are other drawbacks of viral gene carriers[21]. Therefore, non-viral transfection systems based on cationic lipids or cationic polymers have gained more and more attention[22]. Although non-viral carriers may be safer and cheaper, they have, especially in vivo, a much lower transfection efficiency than viral gene carriers. To overcome the limitations of non-viral gene therapy, ultrasound energy, alone or in combination with gas-filled microbubbles, has recently been proposed to enhance the intracellular delivery of DNA, RNA, and proteins[23,24].
Microbubbles and ultrasound have been investigated with a view to improve the transfection efficiency of non-viral delivery systems. Ultrasound can create transient non-lethal perforations in cells and other membranes, which allow large molecules from the surrounding medium to enter into the cell, without remarkable damage so that the cell can reseal its membrane and survive[25,26]. Microbubbles, by acting as cavitation nuclei, applied in combination with the use of ultrasound, are thought to potentiate this effect, particularly effectively for gene delivery[27]. FD500 is the conjugate of fluorescein FITC and dextran with a molecular weight of 500 000, which makes it difficult for FD500 to penetrate the cellular membrane under normal conditions. In this study, HUVECs containing FD500 were treated with ultrasound alone and ultrasound with SonoVue, and the results showed that ultrasound exposure alone induced FD500 (24.0%) into HUVECs, as detected by flow cytometry, and the FD500-positive HUVECs increased to 66.6% after addition of SonoVue. Under ultrasound exposure, microbubbles suspended in liquid can be collapsed intentionally by insonation, and this collapse creates a mechanical force on the cell membrane and destroys the integrity of the adjoining cellular membrane.
Based on the aforementioned findings, we chose the pEGFP to examine the feasibility of gene transfer. GFP, as a report molecule, is used for detecting cytogene expression and protein location. EGFP is the optimized mutant of GFP; the fluorescence it emits is brighter and more sensitive than GFP[28]. The expression of plasmid EGFP in HUVECs was just 1.5% in the group exposed to the ultrasound only, whereas the expression rate significantly increased to 16.1% (P < 0.001) when SonoVue was added, as detected by flow cytometry. The increase in transfection efficiency might be due to the transient holes that are produced in the cell membrane by the spreading of microbubbles. Several studies have reported that gas-body-based contrast agents enhance the non-thermal effects of ultrasound that induce cell membrane porosity[29]. SonoVue seemed to be rapidly destroyed by the sonication, which implies that most of the effects seen were not caused by any direct effect of SonoVue, but by ultrasonic-induced cavitation, which is used to mediate the transfection of the exogenous gene[30]. In the group with the addition of SonoVue only, there was no expression of plasmid EGFP in HUVECs. Compared to the cells treated with ultrasound alone, the addition of SonoVue did not significantly affect cell viability; more than 85% of the cells were viable.
In conclusion, our study demonstrates that ultrasound exposure combined with microbubble significantly improves the transfection efficiency of pEGFP in HUVECs, and the mechanism is based on the enhanced cellular membrane permeability caused by acoustic cavitation with no adverse effect on cellular viability. This method can be used as a new strategy for target gene therapy of HCC.
We thank Dr. Ge Zhang for providing the pEGFP.
It has been shown that ultrasonic waves may increase cell membrane permeability by inducing transient holes in the membrane and facilitate thereby the transfer of DNA into cells and ultrasound-mediated gene transfer is principally due to acoustic cavitation.
Ultrasound-mediated gene transfer can be further enhanced in the presence of microbubble contrast agents.
Tumor angiogenesis plays a critical role in the development and progression of HCC, which is associated with a high propensity for vascular invasion. We attempted to evaluate the effect of microbubble-enhanced ultrasound exposure in improving the transfection of enhanced pEGFP into HUVECs.
The gene therapy method of Microbubble¬-enhanced Ultrasound Exposure on targeting the vascular endothelial cells of HCC has not been used. The aims of the study was to elucidate the mechanism of microbubble-enhanced transfection and to construct a novel gene therapy that might be used to treat HCC.
HUVECs: Human umbilical vein endothelial cells; pEGFP: plasmid DNA encoding enhanced green fluorescent protein.
S- Editor Liu Y L- Editor Kumar M E- Editor Liu WF
| 1. | Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Fernando NH, Hurwitz HI. Targeted therapy of colorectal cancer: clinical experience with bevacizumab. Oncologist. 2004;9 Suppl 1:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706-3712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 491] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 4. | Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF 3rd, Gaudreault J, Damico LA. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1464] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 5. | Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 673] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Ferber D. Gene therapy. Safer and virus-free. Science. 2001;294:1638-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Kaneda Y, Tabata Y. Non-viral vectors for cancer therapy. Cancer Sci. 2006;97:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol. 1996;22:1131-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 342] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Lawrie A, Brisken AF, Francis SE, Tayler DI, Chamberlain J, Crossman DC, Cumberland DC, Newman CM. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999;99:2617-2620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Unger EC, Matsunaga TO, McCreery T, Schumann P, Sweitzer R, Quigley R. Therapeutic applications of microbubbles. Eur J Radiol. 2002;42:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Miller DL, Quddus J. Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast-agent gas bodies. Ultrasound Med Biol. 2000;26:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Brown MD, Schätzlein AG, Uchegbu IF. Gene delivery with synthetic (non viral) carriers. Int J Pharm. 2001;229:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 250] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Ng KY, Liu Y. Therapeutic ultrasound: its application in drug delivery. Med Res Rev. 2002;22:204-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Piconi L, Quagliaro L, Da Ros R, Assaloni R, Giugliano D, Esposito K, Szabó C, Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose) polymerase. J Thromb Haemost. 2004;2:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, Liang JY. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med. 2006;25:23-33. [PubMed] |
| 16. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med. 2006;25:349-361. [PubMed] |
| 17. | Zondervan KT, Cardon LR. The complex interplay among factors that influence allelic association. Nat Rev Genet. 2004;5:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 356] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Liotta LA, Stracke ML. Tumor invasion and metastases: biochemical mechanisms. Cancer Treat Res. 1988;40:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Poon RT, Fan ST, Wong J. Clinical significance of angiogenesis in gastrointestinal cancers: a target for novel prognostic and therapeutic approaches. Ann Surg. 2003;238:9-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:E61-E77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 422] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | Bekeredjian R, Chen S, Grayburn PA, Shohet RV. Augmentation of cardiac protein delivery using ultrasound targeted microbubble destruction. Ultrasound Med Biol. 2005;31:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Kinoshita M, Hynynen K. A novel method for the intracellular delivery of siRNA using microbubble-enhanced focused ultrasound. Biochem Biophys Res Commun. 2005;335:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Fechheimer M, Boylan JF, Parker S, Sisken JE, Patel GL, Zimmer SG. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proc Natl Acad Sci USA. 1987;84:8463-8467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 211] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Tomizawa M, Ebara M, Saisho H, Sakiyama S, Tagawa M. Irradiation with ultrasound of low output intensity increased chemosensitivity of subcutaneous solid tumors to an anti-cancer agent. Cancer Lett. 2001;173:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Nozaki T, Ogawa R, Feril LB Jr, Kagiya G, Fuse H, Kondo T. Enhancement of ultrasound-mediated gene transfection by membrane modification. J Gene Med. 2003;5:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Unger EC, Hersh E, Vannan M, McCreery T. Gene delivery using ultrasound contrast agents. Echocardiography. 2001;18:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 338] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Misteli T, Spector DL. Applications of the green fluorescent protein in cell biology and biotechnology. Nat Biotechnol. 1997;15:961-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 239] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Chen WS, Matula TJ, Crum LA. The disappearance of ultrasound contrast bubbles: observations of bubble dissolution and cavitation nucleation. Ultrasound Med Biol. 2002;28:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Manome Y, Nakayama N, Nakayama K, Furuhata H. Insonation facilitates plasmid DNA transfection into the central nervous system and microbubbles enhance the effect. Ultrasound Med Biol. 2005;31:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |