Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7503
Revised: September 15, 2006
Accepted: September 29, 2006
Published online: December 14, 2006
AIM: To characterize and evaluate the therapeutic efficacy of bioartificial liver (BAL) as compared to that of continuous hemodiafiltration (CHDF) with plasma exchange (PE), which is the current standard therapy for fulminant hepatic failure (FHF) in Japan.
METHODS: Pigs with hepatic devascularization were divided into three groups: (1) a non-treatment group (NT; n = 4); (2) a BAL treatment group (BAL; n = 4), (3) a PE + CHDF treatment group using 1.5 L of normal porcine plasma with CHDF (PE + CHDF, n = 4). Our BAL system consisted of a hollow fiber module with 0.2 μm pores and 1 x 1010 of microcarrier-attached hepatocytes inoculated into the extra-fiber space. Each treatment was initiated 4 h after hepatic devascularization.
RESULTS: The pigs in the BAL and the PE + CHDF groups survived longer than those in the NT group. The elimination capacity of blood ammonia by both BAL and PE + CHDF was significantly higher than that in NT. Aromatic amino acids (AAA) were selectively eliminated by BAL, whereas both AAA and branched chain amino acids, which are beneficial for life, were eliminated by PE + CHDF. Electrolytes maintenance and acid-base balance were better in the CPE + CHDF group than that in the BAL group.
CONCLUSION: Our results suggest that PE + CHDF eliminate all factors regardless of benefits, whereas BAL selectively metabolizes toxic factors such as AAA. However since PE + CHDF maintain electrolytes and acid-base balance, a combination therapy of BAL plus CPE + CHDF might be more effective for FHF.
- Citation: Kawazoe Y, Eguchi S, Sugiyama N, Kamohara Y, Fujioka H, Kanematsu T. Comparison between bioartificial and artificial liver for the treatment of acute liver failure in pigs. World J Gastroenterol 2006; 12(46): 7503-7507
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7503
Fulminant hepatic failure (FHF) has been one of the most challenging problems in clinical medicine. Although plasma exchange (PE) is still a main therapeutic modality in Japan, PE was found not to affect mortality in cases of liver failure[1] . Therefore, recently the combination of continuous PE and continuous hemodiafiltration (CHDF) has been proposed, and its initial clinical results have been encouraging[2-4]. In fact, however, no studies have compared CHDF with other therapeutic modalities.
We have developed a BAL system utilizing porcine hepatocytes cultured on collagen-coated beads and reported on its efficacy[5]. Although many studies have reported the efficacy of CPE + CHDF or BAL treatment, little is known about how these two modalities compare with each other.
The purpose of the present study is to compare the therapeutic efficacy of BAL support for ischemic FHF with that of CPE + CHDF treatment in pigs. In addition, a characterization of the benefit of each treatment was attempted.
All study protocols were reviewed and approved by the University of Nagasaki Research Animal Resources Animal Care Committee and met both institutional and national guidelines.
Hepatocytes were harvested from outbred female white pigs weighing 8 to 10 kg, according to the method described by Seglen with our modification[6]. Briefly, under isoflurane anesthesia, the portal vein was cannulated with a silicon tube and the liver was perfused in situ with 4 L of oxygenated 2 mmol/L ethylenediaminotetraacetic acid (EDTA) solution at the rate of 400 mL/min at 37°C. Immediately after the perfusion, the liver was removed and placed in a sterile basin. Ten minutes later, the EDTA solution was replaced with a Ca2+-enriched Leffert’s buffer solution containing 0.05% type P collagenase (Sigma Chemical Co. St. Louis, MO). The solution was re-circulated through the liver in the basin at a rate of 400 mL/min for 20 min under a clean air-filtered hood. After the collagenase perfusion, the digested liver was manually disrupted in cold DMEM (Nissui Pharmaceutical, Tokyo, Japan) containing 10% heat-inactivated fetal calf serum (FCS, Pharmacia LKB Biotechnology, Piscataway, NJ) and 1% penicillin-streptomycin (Life Technologies, Grand Island, NY). The crude liver cells were filtered through a Mesh Filtration Unit (mesh space, 105 μm; Spectrum, Laguna Hills, CA), and the enriched fractions of the hepatocytes were separated by low-speed centrifugation (at 50 g for 2 min, at 4°C). Centrifugation was performed three times. The initial viability of the hepatocytes was determined using the trypan blue dye exclusion test.
Isolated hepatocytes with a viability of better than 90% and collagen coated dextran microcarrier beads (Cytodex 3, Pharmacia LKB Biotechnology, Piscataway, NJ) were incubated with DMEM with 10% FCS in a cell culture bag (Si culture bag, Wako, Tokyo, Japan) for 4 h at 95% air and 5% CO2 at 37°C. After washing with normal saline, ten billion microcarrier-induced hepatocytes were inoculated into the extra-fiber space (185 mL) of the hollow fiber module with 0.8 m2 of total external fiber surface (Plasmaflo OP-08, Asahi Medical, Tokyo, Japan). The hollow fibers were made from polyethylene materials with an internal diameter of 340 mm, a pore size of 0.2 μm, and membrane thickness of 50 μm.
Under general anesthesia with mechanical ventilation via an endotracheal tube, pigs (weighing 27 to 30 kg) underwent hepatic devascularization consisting of dissection of the biliary tract, hepatic arteries, and ligaments after creation of an end-to-side portocaval shunt according to the method described by Mazziotti et al[7]. This model was reported to reproduce the many pictures of FHF including, encephalopathy, coagulopathy and brain edema. A femoral artery was cannulated for sampling blood and monitoring blood pressure. A jugular vein was also cannulated with a double lumen catheter for blood access. The volume of the replacement fluid was adjusted over the range of 3-6 L, with 5% dextrose lactate Ringer solution depending on the response of the FHF pigs.
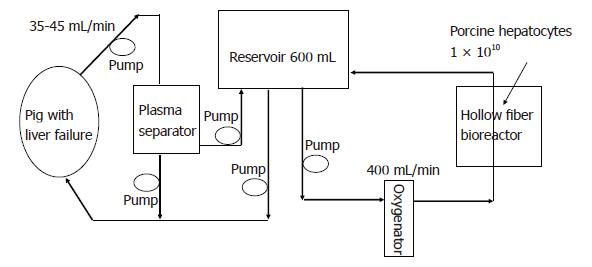
The BAL system consisted of a hollow fiber module inoculated with microcarrier-attached porcine hepatocytes, a plasmapheresis unit (Plasauto 2500 and Plasmaflo OP-2, Asahi Medical, Tokyo, Japan), a roller pump, and a plasma reservoir (Figure 1). The system was oxygenated and warmed at 37°C using a membrane-type oxygenator. Whole blood was removed at a rate of 35-45 mL/min from the jugular vein and was separated to plasma at a rate of 15 mL/min. The separated plasma was perfused through the bioreactor at a rate of 400 mL/min and was recirculated through the whole system with the transmission reservoir (Figure 1).
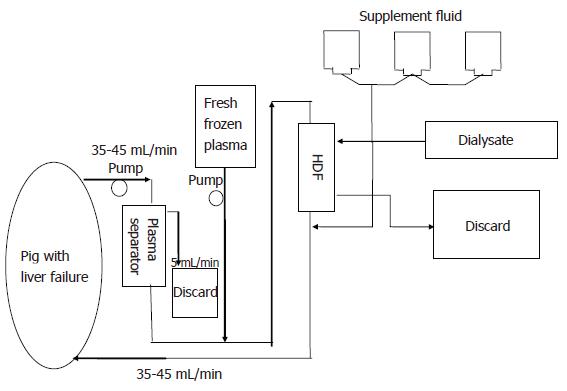
The PE + CHDF system consisted of the plasmapheresis unit mentioned above, a high performance hemodiafiltration unit (Panflo, APF-03S, Asahi Medical,Tokyo, Japan) and three roller pumps (Figure 2). In this system, the apparatuses were connected in a series. PE + CHDF was performed for 6 h. An average of 1.5 L fresh porcine frozen plasma was used during PE treatment. Dialysis was performed concomitantly at a flow rate of 50 mL/min using a dialysis fluid containing bicarbonate buffer (Sublood B; Fuso Pharmaceutical, Tokyo, Japan).
Animals undergoing hepatic devascularization were divided into three groups as follows: a non-treatment group (NT; only plasma separation, n = 4), a BAL treatment group (BAL; n = 4), and a CPE + CHDF treatment group (CPE + CHDF; n = 4). The BAL or CPE + CHDF treatments were initiated at 4 h after completion of hepatic devascularization. The pigs in both the BAL and CPE + CHDF groups were treated for 6 h and were given 800 U/h of heparin as an anticoagulant. After 6 h BAL or CPE + CHDF was stopped and the pigs were observed until their death.
Conventional liver function tests including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), the plasma levels of branched chain amino acids (BCAA), aromatic amino acids (AAA), the ratios of the BCAA to the AAA (Fischer's ratio) after each treatment, and the changes in plasma ammonia were determined. Plasma levels of electrolytes, blood urea nitrogen (BUN), creatinine, and pH were measured. Survival time was also observed.
Data were expressed as mean ± SD, and were analyzed statistically using a non-parametric test. P < 0.05 was considered to indicate a significant difference.
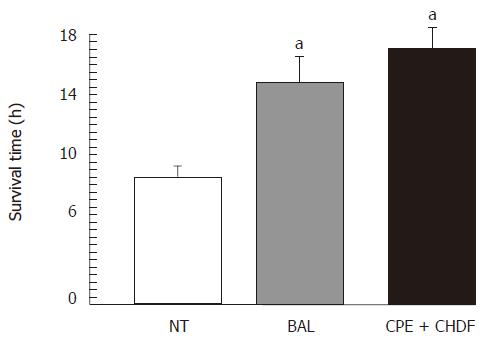
Although the pigs in the BAL and the CPE + CHDF groups survived significantly longer than those in the NT groups, there were no significant differences between the BAL group and the CPE + CHDF group (Figure 3; control: 10.3 ± 1.1 h; BAL: 18.0 ± 1.3 h; CPE + CHDF: 19.2 ± 0.7 h).
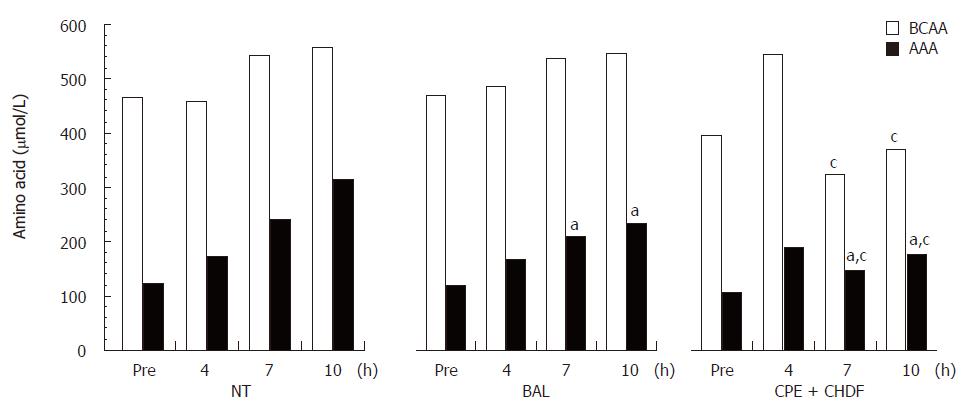
Plasma levels of AST and ALT showed no significant differences among the three experimental groups. The Fischer's ratio was highest in the BAL group (NT: 0.8 ± 0.1; BAL: 1.5 ± 0.3; CPE + CHDF: 1.1 ± 0.2). In the NT group, plasma levels of both AAA and BCAA increased gradually during the treatments. In the BAL groups, whereas AAA levels in plasma decreased significantly, BCAA levels remained unchanged. In contrast, in the CPE + CHDF group, both AAA and BCAA levels in plasma decreased during the treatments (Figure 4).
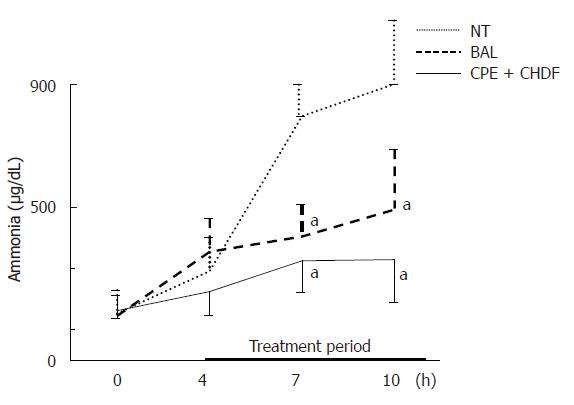
The elimination capacity of ammonia in both the BAL and CPE + CHDF groups was significantly higher than that in the NT group, but there were no significant differences between the BAL and CPE + CHDF groups (Figure 5).
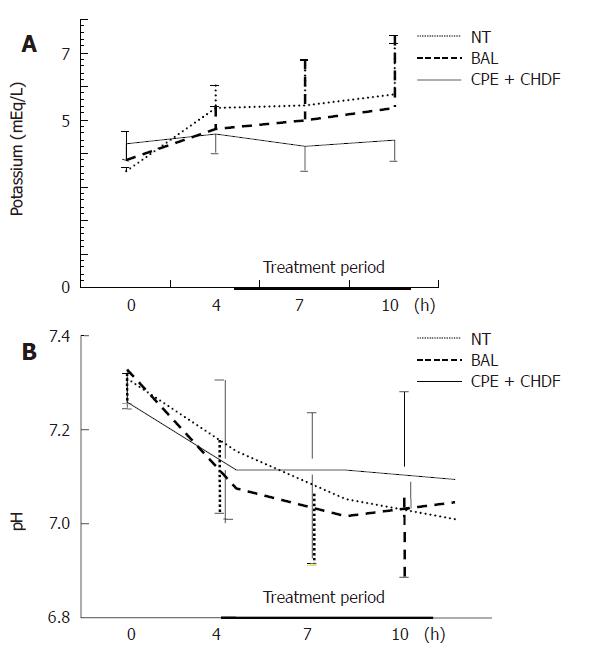
As for electrolyte status, serum potassium level increased with time in both the NT and BAL group. In the CPE + CHDF group, the increase in potassium level was kept within the normal range (Figure 6). As for acid-base balance acidosis deteriorated with time in the NT and BAL group but was rather maintained in the CPE + CHDF group (Figure 6). Although the same pattern among the three groups was also seen, there were no statistical differences between the groups.
Although most nonbiological or biological liver support systems rely on blood detoxification, the removal of medium-sized molecules, which cause hepatic coma, does not improve the survival rate of patients with severe encephalopathy[1]. This implies that the replacement of biotransformation and of liver synthetic functions is also needed in order to maintain life. At present, only intact hepatocytes seem to be able to achieve it[8-10].
In this experiment, the most striking findings for BAL were its effects on changes in plasma ammonia and on amino acids. The elimination capacity of plasma ammonia in BAL and CPE + CHDF was significantly higher than that in NT, although there were no significant differences between BAL and CPE + CHDF. These results indicated that hepatocytes in BAL functioned well and that its ammonia elimination capacity was not inferior to that of the CPE + CHDF treatment. The improvement of Fischer's ratio in BAL treatment was attributable to the decrease in AAA, which is one of the toxic substances in hepatic failure and which is metabolized by intact hepatocytes[11]. It is noteworthy that AAA was selectively eliminated in BAL, whereas both AAA and BCAA, which are beneficial amino acids, were eliminated in CPE + CHDF. This implies that CPE + CHDF eliminated all factors regardless of toxicity or necessity, whereas BAL selectively metabolized toxic factors such as AAA in the blood. To the best of our knowledge, the present results are the first to report the characteristics of two different liver support systems in vivo.
However, as for the maintenance of acid-base balance and electrolytes, CPE + CHDF was superior to BAL. That is, in the model of FHF in pigs, CPE + CHDF outperformed BAL in the maintenance of general status such as acid-base balance and electrolytes abnormalities. In addition, although there were no significant differences between these two groups, the pigs in the CPE + CHDF group survived slightly longer than those in the BAL group. We attributed this to better renal functions and maintenance of acid-base balance in the CPE + CHDF group.
Also, it was possible that CPE + CHDF removed all growth factors that might be needed for a diseased liver to regenerate. This kind of nonselective removal of the indispensable factors has been reported previously by researchers, including ourselves[12]. We showed that, in the rat model undergoing partial hepatectomy, blood exchange therapy reduced the serum level of hepatocyte growth factor significantly, resulting in better liver regeneration with a PCNA labeling index. In this sense, BAL might be valuable in light of its bio-transforming activity, which does not remove all factors needed.
As to survival time, we did not find the difference between the BAL and CPE + CHDF, although both treatments prolonged the survival of pigs with ischemic liver. Therefore, it would be possible to use other models of liver failure such as the one recently published, since our model of liver failure was too aggressive with no chance of spontaneous liver regeneration[13].
In conclusion, we have compared the BAL and CPE + CHDF treatments, especially concerning their elimination capacity. We expect that the combination of BAL and CPE + CHDF would serve as an effective bridging therapy to liver transplantation or liver regeneration in patients with liver failure.
S- Editor Wang GP L- Editor Zhu LH E- Editor Liu WF
| 1. | Freeman JG, Matthewson K, Record CO. Plasmapheresis in acute liver failure. Int J Artif Organs. 1986;9:433-438. [PubMed] |
| 2. | Yoshiba M, Sekiyama K, Iwamura Y, Sugata F. Development of reliable artificial liver support (ALS)--plasma exchange in combination with hemodiafiltration using high-performance membranes. Dig Dis Sci. 1993;38:469-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Nagata Y, Uto H, Hasuike S, Ido A, Hayashi K, Eto T, Hamakawa T, Tanaka K, Tsubouchi H. Bridging use of plasma exchange and continuous hemodiafiltration before living donor liver transplantation in fulminant Wilson's disease. Intern Med. 2003;42:967-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Nakae H, Yonekawa C, Wada H, Asanuma Y, Sato T, Tanaka H. Effectiveness of combining plasma exchange and continuous hemodiafiltration (combined modality therapy in a parallel circuit) in the treatment of patients with acute hepatic failure. Ther Apher. 2001;5:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (5)] |
| 5. | Kamohara Y, Fujioka H, Eguchi S, Kawashita Y, Furui J, Kanematsu T. Comparative study of bioartificial liver support and plasma exchange for treatment of pigs with fulminant hepatic failure. Artif Organs. 2000;24:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 3877] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 7. | Bernardi M, Tacconi C, Somaroli M, Gasbarrini G, Mazziotti A. Hyperammonemic, ammonia-independent coma in experimental acute liver failure induced in the pig. Gastroenterology. 1981;81:191-192. [PubMed] |
| 8. | Rozga J, Podesta L, LePage E, Morsiani E, Moscioni AD, Hoffman A, Sher L, Villamil F, Woolf G, McGrath M. A bioartificial liver to treat severe acute liver failure. Ann Surg. 1994;219:538-544; discussion 544-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 156] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Demetriou AA, Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, Am Esch JS 2nd, Lerut J, Nyberg SL, Salizzoni M. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660-667; discussion 667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 406] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Eguchi S, Lilja H, Hewitt WR, Middleton Y, Demetriou AA, Rozga J. Loss and recovery of liver regeneration in rats with fulminant hepatic failure. J Surg Res. 1997;72:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Shi ZQ, Chang TM. Amino acid disturbances in experimental hepatic coma rats. Int J Artif Organs. 1984;7:197-202. [PubMed] |
| 12. | Eguchi S, Sugiyama N, Kawazoe Y, Kawashita Y, Fujioka H, Furui J, Kanematsu T. Total blood exchange suppresses the early stage of liver regeneration following partial hepatectomy in rats. Artif Organs. 1998;22:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Li LJ, Du WB, Zhang YM, Li J, Pan XP, Chen JJ, Cao HC, Chen Y, Chen YM. Evaluation of a bioartificial liver based on a nonwoven fabric bioreactor with porcine hepatocytes in pigs. J Hepatol. 2006;44:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |