Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7482
Revised: October 25, 2006
Accepted: November 3, 2006
Published online: December 14, 2006
AIM: To explore the effect of gastrin 17 (G17) on β-catenin/T cell factor-4 (Tcf-4) signaling in colonic cancer cell line Colo320WT.
METHODS: The pCR3.1/GR plasmid, which expresses gastrin receptor, cholecystokinin-2 receptor (CCK-2R), was transfected into a colonic cancer cell line Colo320 by Lipofectamine TM2000 and the stably expressing CCK-2R clones were screened by G418. The expression levels of gastrin receptor in the Colo320 and the transfected Colo320WT cell line were assayed by RT-PCR. Colo320WT cells were treated with G17 in a time-dependent manner (0, 1, 6, 12, 24 and 48 h), then with L365,260 (Gastrin17 receptor blocker) for 30 min, and with G17 again for 12 h or L365,260 for 12 h. Expression levels of β-catenin in a TX-100 soluble fraction and TX-100 insoluble fraction of Colo320WT cells treated with G17 were detected by co-immuniprecipation and Western blot. Immunocytochemistry was used to examine the distribution of β-catenin in CoLoWT320 cells. Expression levels of c-myc and cyclin D1 in Colo320WT cells treated with G17 were assayed by Western blot.
RESULTS: Expression levels of β-catenin in the TX-100 solution fraction decreased apparently in a time-dependent fashion and reached the highest level after G17 treatment for 12 h, while expression levels of β-catenin in the TX-100 insoluble fraction were just on the contrary. Immunocytochemistry showed that β-catenin was translocated from the cell membranes into the cytoplasm and nucleus under G17 treatment. Expression levels of c-myc and cyclin D1 in the G17-treated Colo320WT cells were markedly higher compared to the untreated Colo320WT cells. In addition, the aforementioned G17-stimulated responses were blocked by L365,260.
CONCLUSION: Gastrin17 activates β-catenin/Tcf-4 signaling in Colo320WT cells, thereby leading to over-expression of c-myc and cyclin D1.
- Citation: Cao J, Yu JP, Liu CH, Zhou L, Yu HG. Effects of gastrin 17 on β-catenin/Tcf-4 pathway in Colo320WT colon cancer cells. World J Gastroenterol 2006; 12(46): 7482-7487
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7482
Gastrin is a hormone produced by G-cells in the normal gastric antrum. As a peptide hormone and trophic factor, in addition to regulating gastric acid secretion, gastrin exerts a growth-promoting action on gastrointestinal malignancy[1,2]. And there is substantial evidence that gastrin can stimulate the growth and proliferation of some colorectal cancers in vivo and in vitro[3-6]. Colorectal carcinoma cells may also aberrantly produce gastrin. Thus, gastrin may act as an autocrine/paracrine or endocrine factor in initiation and progression of colorectal carcinoma[1]. As yet, some research has shown that gastrin exerts its effect in promoting proliferation and growth by binding its receptor CCK-2[7-11]. We have demonstrated that G17 can cause tyrosine phosphorylation of focal adhesion kinase (FAK), paxillin, and p130Cas in human colon cancer cells. We have also confirmed that G17 may promote colon cancer cell invasion and metastasis by phosphorylating FAKTyr397 and activating FAK pathway[12,13].
The β-catenin/Tcf-4 signaling pathway plays a critical role in gastrointestinal malignancy. As an effector to transmit a receptor-mediated signal from cytosol to the nucleus in the β-catenin/Tcf-4 pathway, β-catenin interacts with and activates the Tcf/Lef transcription factor in the nucleus. The activation of β-catenin/Tcf-4 transcriptional complex can result in expression of multiple target genes, such as c-myc, cyclin D1 and matrilysin, which induce tumor cells invasion and metastasis[14-18]. However, till now, little research has been carried out on whether gastrin exerts its effects on β-catenin/Tcf-4 pathway in colorectal carcinoma cells. We, therefore, aimed to explore the effects of gastrin on β-catenin/Tcf-4 pathway in human colorectal carcinoma cells.
Gastrin-17 amide was purchased from Sigma. The gastrin receptor antagonist L-365,260 and eukaryotic expression vector pCR3.1/GR were kindly provided by St. Josef-Hospital, Ruhr-University Bochum, Germany. Mouse monoclonal antibodies for β-catenin (E-5), E-cadherin (G10), c-myc (9E10), cyclin D1 (A-12) and enhanced chemoluminescence (ECL) reagents were purchased from Santa Cruz Biotechnology. Mouse polyclonal antibodies for Tcf-4 (6H5-3) were obtained from Upstate Biotechnology. Protein G Sepharose 4 Fast Flow and nitrocellulose membranes were obtained from Amersham Pharmacia. Horseradish peroxidase-conjugated anti-mouse secondary antibody was obtained from PIERCR.
Culture of the human colon cancer cell lines Colo320 (ACTCC) was maintained at 37°C in RPMI-1640 medium, supplemented with 100 mL/L fetal bovine serum (FBS) in a humidified atmosphere containing 50 mL/L CO2 and 950 mL/L air. For experimental purposes, cells were plated in 35-mm dishes at a density of 1 × 105 cells per dish and grown in RPMI 1640 medium containing 100 mL/L FBS for 5-7 d.
Colo320 cells were stably transfected with wild-type CCK2 receptor cDNA cloned into the eukaryotic expression vector pCR3.1/GR using the LipofectamineTM2000 according to the manufacturer’s instructions. Following transfection, cells were seeded at very low density to obtain a single cell in an individual well of 96-well plates and further expanded in the presence of 500 mg/mL G418. G418-resistant clones were screened for CCK2 receptor expression by RT-PCR.
Total RNA was extracted from Colo320 and Colo320WT cells by using Trizol reagent. The first strand cDNA was synthesized from 1 μg of total RNA using murine moloney leukemia virus (MuMLV) reverse transcriptase and the first strand cDNA synthesis kit (MBI Fermants) in a total volume of 20 μL. One microliter of each product was subjected to PCR for 30 cycles, each amplification cycle consisting of denaturation at 95°C for 30 s, primers annealing at 60°C for 45 s, and extension at 72°C for 60 s. The primers used were as follows: CCK2, 5’-GTGACAGCGACAGCCAAAGCAG-3’ (sense) and 5’-CGAGGCGTAGCTCAGCAAGTGA-3’ (antisense); β-actin, 5’-CGACGGGAAATCGTGCGTGACATTAAGGAGA-3’ (sense) and 5’-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3’ (antisense). The PCR products were visualized on ethidium bromide-stained 7 g/L agarose gels.
The following procedures were carried out at 4°C as previously described[19-21]. Cells were extracted in the tissue-culture dish with 1 mL of cytoskeleton extraction buffer [CSK: 300 mmol/L, sucrose, 10 mmol/L pipes (pH 6.8), 50 mmol/L NaCl, 3 mmol/L MgCl2, 5 mL/L Triton X-100, 1.2 mmol/L PMSF, 0.1 mg/mL DNase, 0.1 mg/mL RNase]. Cells were harvested with a rubber policeman from the tissue-culture dish, centrifuged at 14 000 g for 10 min and the TX-100-soluble fraction separated from the pellet. The TX-100-insoluble pellet was resuspended in 100 μL of SDS immunoprecipitation buffer [10 g/L SDS, 10 mmol/L Tris-HCl (pH 7.5), 2 mmol/L EDTA, 0.5 mmol/L DTT, 0.5 mmol/L PMSF] and boiled for 10 min. SDS concentration was reduced to 0.1% by the addition of 900 μL of CSK buffer prior to immunoprecipitation. For total cell extract immunoprecipitation, cultures were extracted with 1 mL of lysis buffer [10 mmol/L Tris-HCl (pH 7.5), 1 mmol/L EDTA, 1 mmol/L EGTA, 2 mmol/L PMSF and 5 mL/L Triton X-100] for 10 min and harvested as described above.
The soluble and insoluble fractions were precleared for 1 h by incubation with 1 μg of purified mouse serum IgG and 5 μL of 50% protein G Sepharose at 4°C. Following centrifugation at 3000 r/min for 15 min, the TX-100-soluble fraction was processed for immunoprecipitation with 1 µg of E-cadherin antibodies and 20 μL of 50% protein G Sepharose overnight at 4°C. TX-100-insoluble fractions were processed for immunoprecipitation with 1 µg of β-catenin antibodies and 20 μL of 50% protein G Sepharose overnight, because TX-100-insoluble fractions have to be solubilized prior to immunoprecipitation and the solubilization conditions (10 g/L SDS at 100°C) caused dissociation of the complex. Purified mouse serum Ig was used as a negative control. The beads were washed sequentially with high stringency buffer [15 mmol/L Tris-HCL (pH 7.5), 5 mmol/L EDTA, 2.5 mmol/L EGTA, 10 mL/L TX-100, 10 g/L sodium deoxycholate, 1 g/L SDS, 120 mmol/L NaCl and 25 mmol/L KCl], high-salt buffer [15 mmol/L Tris-HCl (pH 7.5), 5 mmol/L EDTA, 2.5 mmol/L EGTA, 10 mL/L TX-100, 10 g/L sodium deoxycholate, 1 g/L SDS and 1 mol/L NaCl] and low-salt buffer [15 mmol/L Tris-HCl (pH 7.5), and 5 mmol/L EDTA]. The beads were then resuspended in an equal volume of sample buffer, boiled for 5 min and centrifuged at 14 000 g for 5 min, and the supernatant was dissolved by SDS-PAGE on an 80 g/L polyacrylamide gel and transferred by electroblotting onto nitrocellulose membrane and probed with anti-β-catenin antibodies. For β-catenin/Tcf-4 complex coimmunoprecipitation, the total cell extract was precipitated with anti-Tcf-4 antibody and the immunoprecipitated proteins were subjected to probe with anti-β-catenin or anti-Tcf-4, respectively.
Cells were grown to approximately 80% confluence on tissue-culture multispot glass microscope slides at 37°C in an incubator containing 50 mL/L CO2 in absence of serum. The next day, cells were treated with 10-8 mol/L G17 or with 10-6 mol/L CCK2 receptor antagonist L365,260 for 30 min, followed by treatment with G17 for 12 h. The cells were then fixed in acetone for 10 min at 4°C. Endogenous peroxidase was blocked by incubating the slides in 3 mL/L hydrogen peroxide (H2O2) in phosphate-buffered saline (PBS) for 15 min. The slides were incubated in normal goat serum for 20 min to block the non-specific binding. Primary antibody was added to the cells (β-catenin, 1:50), and the slides were incubated overnight at 4°C. The cells were washed in PBS and then appropriate biotinylated secondary antibody was added to each well. The slides were incubated for 30 min at room temperature, washed in PBS, and then incubated with streptavidin-HRP for 30 min at room temperature. The slides were examined by a conventional light microscope, and cellular distributions of the proteins between the membrane, cytoplasm and nucleus were assessed.
Cells were grown to approximately 80% confluence and then serum-starved for 24 h. Then the cells were treated with 10-8 mol/L G17 or with 10-6 mol/L CCK2 receptor antagonist L365,260 as described above. The stimulation was terminated on ice by aspirating the medium and solubilizing the cells in 1 mL of ice-cold RIPA buffer (10 mL/L NP-40, 1% DOC, 1 g/L SDS, 150 mmol/L NaCL, 10 mmol/L Tris-HCl, 1 μmol/L PMSF, 1 μg/mL leupeptin, 1 μg/mL Aprotinin, 1 μg/mL Pepstatin). Cell lysates were centrifuged at 14 000 g for 5 min. The supernatants were transferred into new ice-cold micro-centrifuge tubes. Following SDS-PAGE, proteins were transferred onto nitrocellulose membranes. For detection of proteins, membranes were blocked using 50 g/L non-fat dried milk in Tris buffer containing 1 g/L Tween (TBST) and then incubated overnight at 4°C with specific antibodies diluted in TBS-T containing 50 g/L non-fat milk. Bound primary antibodies to immunoreactive bands were visualized by ECL detection with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies.
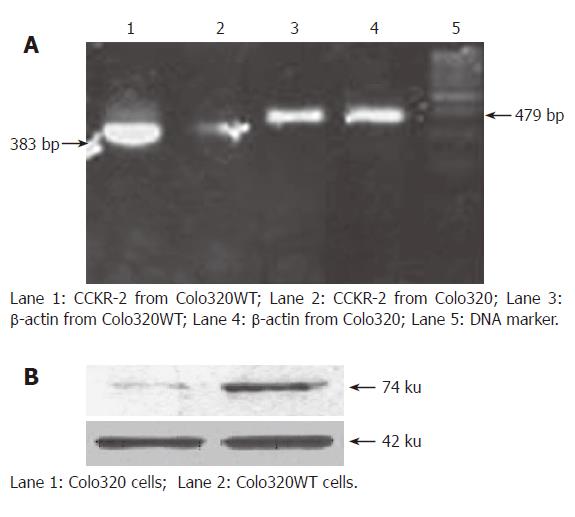
The transfection efficiencies in Colo320 and Colo320 WT cells were evaluated by RT-PCR and immunoblotting. Although no method is capable of discriminating between endogenous and transfected CCK2 receptor expressions, our results of RT-PCR and immunoblotting showed that Colo320 cells expressed low levels of CCK2 receptor mRNA and protein, and that stable transfection with CCK2 receptor cDNA led to a 4-fold over-expression of the CCK2 receptor at protein and mRNA levels (Figure 1A and B).
β-catenin in the TX-100 solution fraction was precipitated with anti-E-cadherin antibody. Expression of β-catenin in the TX-100 solution fraction increased in a time-dependent fashion under G17 treatment, and the expression level reached the highest when treated for 12 h. While the expression of β-catenin in the TX-100 insoluble fraction was obviously decreased, and β-catenin in free pool increased (Figure 2).
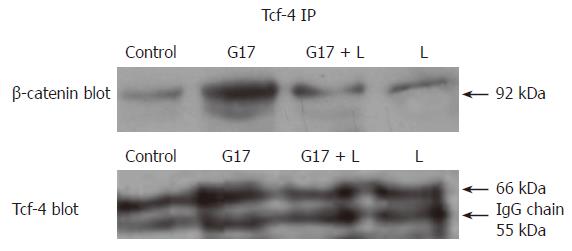
β-catenin interacts with Tcf-4 and forms a complex in the nuclei. We used anti-Tcf-4 antibody to precipitate the complex in the total cell extract, and then go on to probe by anti-β-catenin or anti-Tcf-4 antibody, respectively. As a result, we found that the expression levels of β-catenin and Tcf-4 proteins in the complex increased greatly after G17 stimulation, which confirmed that more β-catenin translocated into the nucleus where it bound and interacted with Tcf-4. The active β-catenin/Tcf-4 transcriptional complex further resulted in the expression of the target genes, such as c-myc and cyclin D1 (Figure 3).
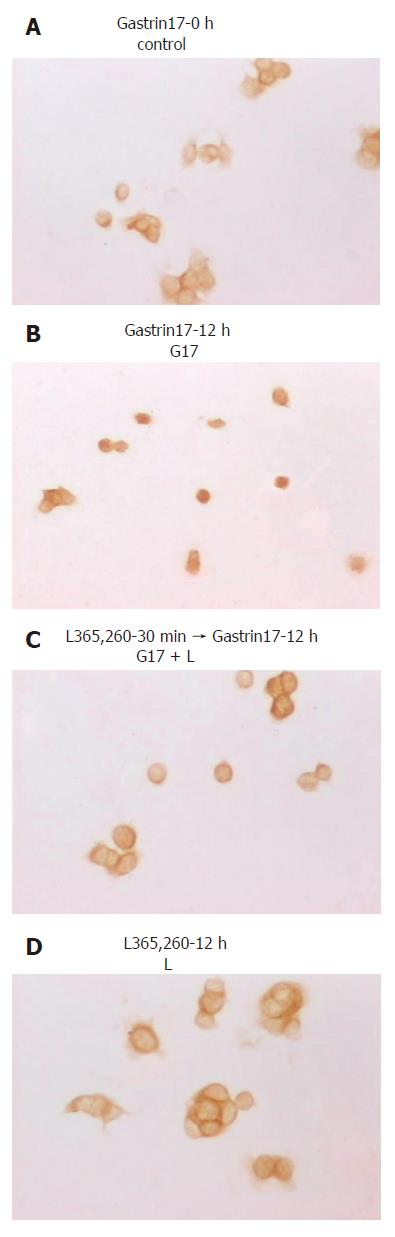
Before treatment with gastrin, scattered Colo320WT cells showed cytoplasmic and little nuclear immunoreactivity for β-catenin in the absence of serum. When confluent, Colo320WT cell membrane localization of β-catenin was at sites of cell-cell contact, but free borders of the cells showed little membranous staining. After G17 stimulation, distribution of β-catenin in the cells changed greatly and β-catenin was translocated from the membrane to the cytoplasm and nucleus, especially to scattered cells. In cohesive cells, staining decreased at sites of cell-cell contact. But after application of L365,260, straining of β-catenin in the cells was the same as that of the cells untreated by G17 (Figure 4).
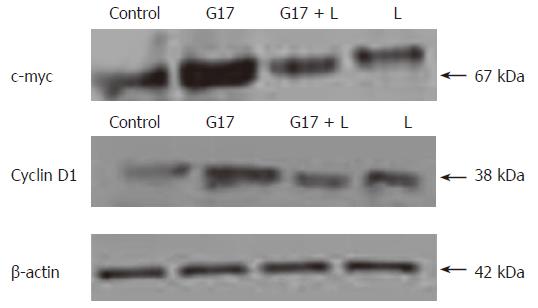
After stimulating Colo320WT cells by G17, expression levels of c-myc and cyclin D1 protein were greatly increased, showing that gastrin can translocate β-catenin into the nucleus where it interacts with Tcf-4, resulting in over-expression of downstream molecules c-myc and cyclin D1, which may cause tumor cell invasion and metastasis (Figure 5).
The gastrointestinal (GI) peptide hormone gastrin has been shown to regulate multiple cellular functions, including growth, apoptosis and secretion[22]. Many studies have also shown that gastrin can stimulate the growth and proliferation of colorectal cancer cells from animals and human[3-6] and gastrin exerts its effects in promoting proliferation and growth by binding its high affinity receptor CCK-2, which belongs to the family of G protein-coupled receptors[7-11]. The gastrin receptor antagonist can abrogate responses stimulated by gastrin[23,24]. We have confirmed that G17 may increase invasion of human colorecatal cancer cell by activating the FAK pathway[12,13]. Recently, we have also found that G17 can lead to phosphorylated FAKTyr397 to accumulate at lamellipodia and to form the FAK-Src-p130Cas-Dock180 signaling complex in human colorectal cancer cells (unpublished data).
β-catenin is a member of the catenin family as a proto-oncogene. Adenomatous polyposis coli (APC) can compete with E-cadherin to bind and interact with β-catenin. β-catenin binds the intracellular domain of E-cadherin and forms a complex, which keeps low levels of β-catenin in the cytoplasm. Alpha-catenin mediates the anchorage of the E-cadherin/β-catenin complex to actin filaments of the cytoskeleton and participates in cytoskeleton remodeling[25-28]. Cytosolic β-catenin is degraded by the ubiquitin-proteasome pathway, requiring active glycogen synthase kinase-3β (GSK-3β), APC and axin[25,29-31].
Our study showed that G17 could stimulate redistribution of β-catenin in human colon cancer cells Colo320WT, which is different from the results achieved by Song et al[32] that gastrin can lead only to high expression of β-catenin, but not redistribution of β-catenin in mouse colorectal cells. The different results probably resulting from different genus cells (mouse and human) are to be studied in our following research. Our coimmunoprecipation results showed that the expression level of β-catenin in the TX-100 insoluble fraction markedly increased in a time-dependent manner, and the expression level of β-catenin reached the highest when G17 was exogenously applied for 12 h. But the expression level of β-catenin in the TX-100 soluble fraction was the other way around. It indicates that free cytosolic β-catenin increases greatly under G17 treatment, because G17 may inhibit activation of APC, axin, PTEN (the phosphatase and tensin homologue) and GSK-3β, and further inhibit β-catenin phosphorylation. As a result, free β-catenin in the cytoplasmic pool increases on gastrin stimulation. When we used L365,260 (gastrin receptor antagonist) to block effects caused by G17, expression level of β-catenin changed a little in the TX-100-insoluble fraction and the TX-100-soluble fraction, which confirmed that G17 exerts its effects on Colo320WT cells by binding and interacting with its high-affinity receptor CCK-2R.
Immunocytochemistry demonstrated a link between cell-cell contact and the distribution of β-catenin. Without G17 stimulation, scattered Colo320WT cells showed cytoplasmic and little nuclear immunorectivity for β-catenin, and the confluent Colo320WT cells membrane localization of β-catenin was at sites of cell-cell contact, but free borders of the cells showed little membranous staining. But after G17 stimulation, distribution of β-catenin in the cells changed greatly and β-catenin was translocated from the membrane to the cytoplasm and nucleus, especially to scattered cells. In cohesive cells, staining decreased at sites of cell-cell contact. Taken together, G17 can affect redistribution of β-catenin in Colo320WT cells and decrease cell-cell cohesion, resulting in cell invasion and metastasis.
G17 leading to redistribution of β-catenin in Colo320WT cells resulted in an increase of the cytoplasmic pool of β-catenin. The increased free β-catenin was translocated into the nucleus, where it bound and interacted with Tcf-4 transcription factor. Thus, activated β-catenin/Tcf-4 pathway led to the up-regulation of downstream target genes c-myc and cyclin D1.
In conclusion, G17 can cause redistribution of β-catenin and activate β-catenin/Tcf-4 pathway which leads to high expression of c-myc and cyclin D1, thereby promoting invasion and metastasis of Colo320WT cells.
Colorectal cancer is one of human malignant tumor, and tumor metastasis affects therapeutic effect and prognosis, playing a key role in death to a patient with cancer. But the biologic mechnism of tumor cells’ motility, invasion and metastasisis to be studied as yet.
Gastrin may mediate intracellular signal transduction in human colon cancer cells, and can increase invasion and metastasis of tumor cells by binding to its receptor CCK-2.
Our study shows that Gastrin17 may induce redistribution of β-catenin in human colon cancer cells Colo320WT, which is different from the results achieved by Song DH et al that gastrin can lead only to high expression of β-catenin, but not redistribution of β-catenin in mouse colorectal cells.
The research explores the mechanism of colorectal cancer invasion and metastasis in order to provide a new thinking of preventing and curing tumor invasion and metastasis.
Beta -catenin in the TX-100 solution fraction is cytoplasmic beta -catenin, while beta -catenin in TX-100 insoluble fraction is cytoskeleton bound beta -catenin.
Gastrin is a peptidic hormone essentially secreted by gastric antrum and proximal duodenum, which belongs to the same family as cholecystokinin (CCK). More recent findings suggest that gastrin can mediate proliferative effects in digestive tract neoplasia by the CCK2 receptor. And some clinical evidence and animal experiments have shown that gastrin may promote tumor’s invasivness and metastasis, regrettably, its mechanism is still to be explored as yet.
The experiment aims to explore the mechanism by which gastrin increases tumor’s invasion and metastasis by plasmid transfection, RT-PCR, coimmunoprecipitation and Western blot methods. The results showed that G17 induced redistribution of β-catenin and increased free cytoplasmic β-catenin which translocated into the nucleus, where it was bound and interacted with Tcf-4 transcription factor. G17 activated the β-catenin/Tcf-4 pathway, and further upregulated downstream target genes c-myc and cyclinD1.
This article is clear-cut and easy to understand. The paper provides new thoughts on how gastrin affects gastrointestinal tract tumorigenesis, development, invasion and metatasis.
S- Editor Liu Y L- Editor Kumar M E- Editor Liu WF
| 1. | Guo YS, Cheng JZ, Jin GF, Gutkind JS, Hellmich MR, Townsend CM Jr. Gastrin stimulates cyclooxygenase-2 expression in intestinal epithelial cells through multiple signaling pathways. Evidence for involvement of ERK5 kinase and transactivation of the epidermal growth factor receptor. J Biol Chem. 2002;277:48755-48763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol. 2001;63:49-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Yao M, Song DH, Rana B, Wolfe MM. COX-2 selective inhibition reverses the trophic properties of gastrin in colorectal cancer. Br J Cancer. 2002;87:574-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Watson SA, Morris TM, McWilliams DF, Harris J, Evans S, Smith A, Clarke PA. Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Br J Cancer. 2002;87:567-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Ahmed S, Budai B, Herédi-Szabó K, Farkas J, Tóth G, Murphy RF, Lovas S. High and low affinity receptors mediate growth effects of gastrin and gastrin-Gly on DLD-1 human colonic carcinoma cells. FEBS Lett. 2004;556:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Haigh CR, Attwood SE, Thompson DG, Jankowski JA, Kirton CM, Pritchard DM, Varro A, Dimaline R. Gastrin induces proliferation in Barrett's metaplasia through activation of the CCK2 receptor. Gastroenterology. 2003;124:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Clerc P, Leung-Theung-Long S, Wang TC, Dockray GJ, Bouisson M, Delisle MB, Vaysse N, Pradayrol L, Fourmy D, Dufresne M. Expression of CCK2 receptors in the murine pancreas: proliferation, transdifferentiation of acinar cells, and neoplasia. Gastroenterology. 2002;122:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Dehez S, Daulhac L, Kowalski-Chauvel A, Fourmy D, Pradayrol L, Seva C. Gastrin-induced DNA synthesis requires p38-MAPK activation via PKC/Ca(2+) and Src-dependent mechanisms. FEBS Lett. 2001;496:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Bierkamp C, Kowalski-Chauvel A, Dehez S, Fourmy D, Pradayrol L, Seva C. Gastrin mediated cholecystokinin-2 receptor activation induces loss of cell adhesion and scattering in epithelial MDCK cells. Oncogene. 2002;21:7656-7670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657-20663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Yu HG, Schrader H, Otte JM, Schmidt WE, Schmitz F. Rapid tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130Cas by gastrin in human colon cancer cells. Biochem Pharmacol. 2004;67:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Ding J, Yu JP, Li D, Yu HG, Luo HS, Wei WZ. [Effect of gastrin on invasiveness of human colon cancer cells]. Zhonghua Zhong liu Zazhi. 2005;27:213-215. [PubMed] |
| 14. | Fujita M, Furukawa Y, Tsunoda T, Tanaka T, Ogawa M, Nakamura Y. Up-regulation of the ectodermal-neural cortex 1 (ENC1) gene, a downstream target of the beta-catenin/T-cell factor complex, in colorectal carcinomas. Cancer Res. 2001;61:7722-7726. [PubMed] |
| 15. | Giarré M, Semënov MV, Brown AM. Wnt signaling stabilizes the dual-function protein beta-catenin in diverse cell types. Ann N Y Acad Sci. 1998;857:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522-5527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1701] [Cited by in RCA: 1781] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 17. | van de Wetering M, Barker N, Harkes IC, van der Heyden M, Dijk NJ, Hollestelle A, Klijn JG, Clevers H, Schutte M. Mutant E-cadherin breast cancer cells do not display constitutive Wnt signaling. Cancer Res. 2001;61:278-284. [PubMed] |
| 18. | Brabletz T, Herrmann K, Jung A, Faller G, Kirchner T. Expression of nuclear beta-catenin and c-myc is correlated with tumor size but not with proliferative activity of colorectal adenomas. Am J Pathol. 2000;156:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | El-Bahrawy M, Poulsom R, Rowan AJ, Tomlinson IT, Alison MR. Characterization of the E-cadherin/catenin complex in colorectal carcinoma cell lines. Int J Exp Pathol. 2004;85:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Reuver SM, Garner CC. E-cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. J Cell Sci. 1998;111:1071-1080. [PubMed] |
| 21. | Jansson EA, Are A, Greicius G, Kuo IC, Kelly D, Arulampalam V, Pettersson S. The Wnt/beta-catenin signaling pathway targets PPARgamma activity in colon cancer cells. Proc Natl Acad Sci USA. 2005;102:1460-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Pradeep A, Sharma C, Sathyanarayana P, Albanese C, Fleming JV, Wang TC, Wolfe MM, Baker KM, Pestell RG, Rana B. Gastrin-mediated activation of cyclin D1 transcription involves beta-catenin and CREB pathways in gastric cancer cells. Oncogene. 2004;23:3689-3699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Watson SA, Clifford T, Sykes RE, Robinson E, Steele RJ. Gastrin sensitivity of primary human colorectal cancer: the effect of gastrin receptor antagonism. Eur J Cancer. 1995;31A:2086-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Todisco A, Takeuchi Y, Urumov A, Yamada J, Stepan VM, Yamada T. Molecular mechanisms for the growth factor action of gastrin. Am J Physiol. 1997;273:G891-G898. [PubMed] |
| 25. | Oloumi A, McPhee T, Dedhar S. Regulation of E-cadherin expression and beta-catenin/Tcf transcriptional activity by the integrin-linked kinase. Biochim Biophys Acta. 2004;1691:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Davies G, Jiang WG, Mason MD. Matrilysin mediates extracellular cleavage of E-cadherin from prostate cancer cells: a key mechanism in hepatocyte growth factor/scatter factor-induced cell-cell dissociation and in vitro invasion. Clin Cancer Res. 2001;7:3289-3297. [PubMed] |
| 27. | Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 326] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Beavon IR. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer. 2000;36:1607-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 271] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797-3804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1938] [Cited by in RCA: 2036] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 30. | D'Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, Fu M, Augenlicht LH, Donehower LA, Takemaru K. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem. 2000;275:32649-32657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 194] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Gottardi CJ, Gumbiner BM. Adhesion signaling: how beta-catenin interacts with its partners. Curr Biol. 2001;11:R792-R794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Song DH, Kaufman JC, Borodyansky L, Albanese C, Pestell RG, Wolfe MM. Gastrin stabilises beta-catenin protein in mouse colorectal cancer cells. Br J Cancer. 2005;92:1581-1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |