Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7463
Revised: September 16, 2006
Accepted: September 25, 2006
Published online: December 14, 2006
Apoptosis is necessary for maintaining the integrity of proliferative tissues, such as epithelial cells of the gastrointestinal system. The role of apoptosis in post mitotic tissues, such as skeletal muscle, is less well defined. Apoptosis during muscle atrophy occurs in both myonuclei and other muscle cell types. Apoptosis of myonuclei likely contributes to the loss of muscle mass, but the mechanisms underlying this process are largely unknown. Caspase-dependent as well as -independent pathways have been implicated and the mode by which atrophy is induced likely determines the apoptotic mechanisms that are utilized. It remains to be determined whether a decrease in apoptosis will alleviate atrophy and distinct research strategies may be required for different causes of skeletal muscle loss.
- Citation: Dupont-Versteegden EE. Apoptosis in skeletal muscle and its relevance to atrophy. World J Gastroenterol 2006; 12(46): 7463-7466
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7463.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7463
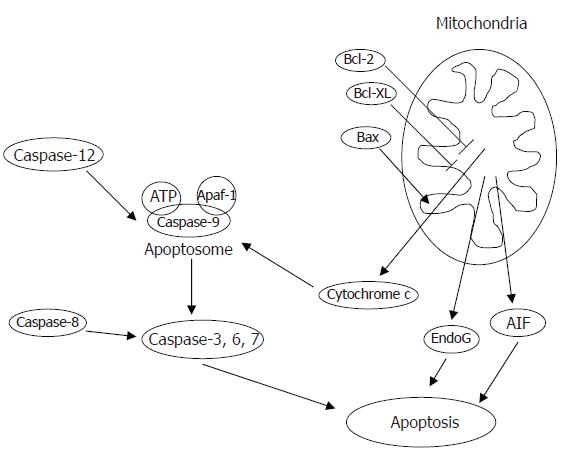
Apoptosis, or programmed cell death, is an important process in multicellular organisms both during development, where it ensures the elimination of superfluous tissues, and in adulthood, where it is critical for maintenance of tissue homeostasis. The early stage of apoptosis involves death-inducing signals, such as reactive oxygen and nitrogen species, ligands for the death receptors, imbalances in calcium regulation, and alterations in the composition and abundance of B-cell lymphoma (Bcl)-2 family proteins, such as Bax, Bad, Bcl-2, Bcl-xl (see[1] for review). After this induction phase, nuclear activators, cell surface receptors, or mitochondrial pathways become activated in the commitment to cellular death followed by cytoplasmic and nuclear events. During apoptosis, protein-cleaving enzymes, i.e., caspases (cysteine-dependent aspartate-directed proteases), become activated in the cytosol and are responsible for proteolytic cleavage of a broad spectrum of cellular targets[2]. Caspase-independent mechanisms also exist, such as the release of apoptosis-inducing factor (AIF) and endonuclease G (EndoG) from mitochondria (Figure 1), inducing large scale DNA fragmentation and apoptosis after translocation to the nucleus[3-5]. In the nucleus, DNA fragmentation caused by activated endonucleases, chromatin condensation, and the breakdown of the nuclear envelope occurs and eventually the cell itself disintegrates into apoptotic bodies and is phagocytosed by surrounding cells or macrophages[6]. Both extrinsic or intrinsic stimuli can be responsible for the induction of apoptosis, with some cross-talk between signaling pathways[1,7,8].
In highly proliferative tissues, such as the intestinal epithelium, apoptosis serves to maintain a constant number of cells and consistent tissue architecture, counterbalancing the rapid proliferation. Indeed, cell loss in the normal intestine can largely be explained by apoptosis[9]. In addition, in the small intestine apoptosis serves to stabilize the stem cell population by removing excess, or perhaps compromised, stem cells[10]. Decreased rates of apoptosis have been observed during tumor progression in colon carcinomas[11] and the overall decrease in apoptosis in proliferative tissues may predispose the cells in those tissues to accumulate genetic changes characteristic of tumorigenesis.
In contrast, in post mitotic tissues such as skeletal muscle, the role of apoptosis is less clear. Also, with respect to cell death, skeletal muscle is a unique tissue because muscle cells, i.e., myofibers, are multinucleated. This aspect of skeletal muscle has led to the concept of the myonuclear domain, which is defined as the theoretical amount of cytoplasm supported by a single muscle fiber nucleus, which is called a myonucleus (protein/DNA)[12]. Even though muscle size can vary considerably under different conditions, the myonuclear domain size remains relatively constant, implying a fairly strict regulation of myonuclear number (for review[13]). Myonuclear number decreases in muscles undergoing atrophy in a variety of experimental conditions, such as spinal cord isolation and transection, microgravity, hind limb suspension, and chronic denervation[14-20] and the process by which nuclei are eliminated from muscle fibers resembles apoptosis. Since destruction of the entire cell does not necessarily follow the elimination of a nucleus, as occurs with apoptosis in mononucleated cells, this process is called ‘apoptotic nuclear death’. The mechanisms underlying apoptotic nuclear death in muscle are likely distinct from those involved in apoptosis in mononucleated cells.
The fact that apoptosis plays an important role in skeletal muscle atrophy can be deduced from the observation that it is increased in skeletal muscle in a number of pathological and under some physiological circumstances. Chronic heart failure, motor neuron disorders, skeletal muscle denervation, spinal cord injury, muscular dystrophy, and skeletal muscle atrophy due to hind limb suspension or immobilization are all associated with an increase in apoptosis in affected skeletal muscles, as measured by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) or by DNA fragmentation in gel electrophoresis[16,21-26]. In addition, exercise was shown to increase apoptosis when assayed acutely after a bout of exercise[24], but by contrast, exercise training for a period of 8 weeks decreased apoptosis[27]. Interestingly, we and others have shown that exercise training attenuated the apoptosis induced by disuse (spinal cord injury or hind limb suspension)[16,26]. This exercise-associated decrease in apoptosis under atrophy-inducing conditions may depend on the mode or intensity of exercise, since recently we found that gravity-independent resistance exercise did not decrease apoptosis during hind limb suspension[28]. Therefore, apoptosis in skeletal muscle seems to be a highly regulated process that may serve distinct functions under different physiological and pathological conditions and a better understanding of pathways involved in the apoptotic response in muscle is warranted.
Caspases are thought to be the main enzymes involved in both the initiation (caspase-8, -9, -12) and execution of apoptosis (caspase-3, -6, -7) (Figure 1). Internal as well as external signals can activate caspases and this is under the control of the balance between pro-and anti-apoptotic proteins of the Bcl-2 family, heat shock proteins, and inhibitors of apoptosis proteins (IAPs)[29]. However, apoptosis can ensue in the absence of caspase activation, whereas caspase activation does not always necessarily trigger cell death[30]. Caspase-3 is often used as a surrogate for apoptosis, but in muscle, in particular, this may not be justified. We and others found that caspase-3 activity did not increase with atrophy induced by hind limb suspension[31,32], even though apoptosis was increased concurrently, indicating that other pathways, besides caspase-3 activation, may be involved. In contrast, denervation-induced atrophy was associated with an increase in caspase-3 activity[33] as well as caspase-8[34], indicating that the activation of caspases may serve a different role in distinct models of muscle atrophy or that the mode by which atrophy occurs determines the involvement of different pathways. Indeed, caspases have been found to play non-apoptotic roles in pathways such as the inflammatory response, immune cell proliferation and differentiation of various cell types (e.g. skeletal muscle)[35]. In skeletal muscle, caspase-3 was found to be involved in protein degradation, in particular of filamentous actin[36], and it contributed to muscle weakness in response to endotoxin[37]. Similarly, in cardiac muscle caspase-3 was shown to be involved in post-ischemic contractile dysfunction (cardiac stunning), apparently independent of apoptosis[38]. Therefore, classical apoptotic pathways, as observed in mononucleated cells, may not be as important in muscle and other molecules may take on the role of apoptosis inducers.
In this light, we have investigated the role of EndoG during skeletal muscle atrophy[31,39]. EndoG is a protein released from mitochondria upon pro-apoptotic stimulation and is capable of inducing DNA fragmentation independent of caspase activation[40-42] (Figure 1). We found that EndoG co-localization with nuclei was increased in muscles atrophied in response to both hind limb suspension and age[31,39] and that EndoG protein was increased in muscles of aged rats undergoing disuse-induced atrophy[31]. Moreover, EndoG translocation was very specific for myonuclei and did not seem to be involved in the apoptosis of interstitial cells, which also occurs during muscle atrophy[39]. In contrast, caspase-3 activation was observed more in interstitial cells and therefore different cell types within the same tissue may be undergoing apoptosis through different mechanisms. Another protein released from mitochondria upon pro-apoptotic stimulation and capable of inducing apoptosis independent of caspases is apoptosis inducing factor (AIF)[43]. Siu and Alway[33] showed that AIF release was elevated in denervated muscle, in concert with cytochrome-c, Smac/DIABLO and a subsequent upregulation of caspase-9 and -3. Therefore, the atrophy-inducing stimulus, the time point after onset of disuse, or the different cell types undergoing apoptosis may be important in the activation of distinct apoptotic pathways. Finding interventions to counteract the increase of apoptosis with muscle atrophy will be challenging, considering the many different pathways used to induce apoptosis in skeletal muscle.
The question arises whether inhibiting apoptosis in skeletal muscle will also decrease atrophy induced by disuse or due to aging. Recently, Siu and Alway[44] showed that inhibition of apoptosis somewhat attenuated muscle atrophy induced by denervation in a Bax knock out mouse model. Interestingly, DNA fragmentation after denervation was much lower in the knock out mice compared to the wild type and caspase activation was also lower. However, mitochondrial AIF release was not decreased, possibly implying that caspase-independent mechanisms were not affected. In this study, the effect on different cell types was not investigated, but it is plausible that the minimal effect on muscle mass could be due to the fact that myonuclear apoptotic loss was not affected by the Bax-/- phenotype. Therefore, it remains to be determined whether inhibition of myonuclear apoptosis will decrease muscle atrophy.
Recently a number of studies have implicated an important role for apoptosis in the development of skeletal muscle atrophy. It is likely that atrophy induced by different conditions, such as denervation, microgravity, aging, cachexia, and spinal cord injury, initiates different apoptotic signals and indeed the role of apoptosis may be distinct among the conditions. It will be important to investigate, at the cellular level, which signals are responsible for the loss of myonuclei, interstitial cells, or stem cells in skeletal muscle in order to develop strategies to decrease apoptosis and atrophy.
S- Editor Liu Y L- Editor Lutze M E- Editor Bi L
| 1. | Primeau AJ, Adhihetty PJ, Hood DA. Apoptosis in heart and skeletal muscle. Can J Appl Physiol. 2002;27:349-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 2002] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 3. | Candé C, Vahsen N, Garrido C, Kroemer G. Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ. 2004;11:591-595. [PubMed] |
| 4. | Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1176] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 5. | van Loo G, Schotte P, van Gurp M, Demol H, Hoorelbeke B, Gevaert K, Rodriguez I, Ruiz-Carrillo A, Vandekerckhove J, Declercq W. Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ. 2001;8:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann N Y Acad Sci. 2002;959:93-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Mayer B, Oberbauer R. Mitochondrial regulation of apoptosis. News Physiol Sci. 2003;18:89-94. [PubMed] |
| 8. | Dupont-Versteegden EE. Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol. 2005;40:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569-3577. [PubMed] |
| 10. | Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Sinicrope FA, Roddey G, McDonnell TJ, Shen Y, Cleary KR, Stephens LC. Increased apoptosis accompanies neoplastic development in the human colorectum. Clin Cancer Res. 1996;2:1999-2006. [PubMed] |
| 12. | Cheek DB. The control of cell mass and replication. The DNA unit--a personal 20-year study. Early Hum Dev. 1985;12:211-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol (1985). 1995;78:1969-1976. [PubMed] |
| 15. | Dupont-Versteegden EE, Murphy RJ, Houlé JD, Gurley CM, Peterson CA. Mechanisms leading to restoration of muscle size with exercise and transplantation after spinal cord injury. Am J Physiol Cell Physiol. 2000;279:C1677-C1684. [PubMed] |
| 16. | Dupont-Versteegden EE, Murphy RJ, Houlé JD, Gurley CM, Peterson CA. Activated satellite cells fail to restore myonuclear number in spinal cord transected and exercised rats. Am J Physiol. 1999;277:C589-C597. [PubMed] |
| 17. | Allen DL, Yasui W, Tanaka T, Ohira Y, Nagaoka S, Sekiguchi C, Hinds WE, Roy RR, Edgerton VR. Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J Appl Physiol (1985). 1996;81:145-151. [PubMed] |
| 18. | Allen DL, Linderman JK, Roy RR, Grindeland RE, Mukku V, Edgerton VR. Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J Appl Physiol (1985). 1997;83:1857-1861. [PubMed] |
| 19. | Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE. Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol (1985). 2004;97:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Rodrigues Ade C, Schmalbruch H. Satellite cells and myonuclei in long-term denervated rat muscles. Anat Rec. 1995;243:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Adams V, Jiang H, Yu J, Möbius-Winkler S, Fiehn E, Linke A, Weigl C, Schuler G, Hambrecht R. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol. 1999;33:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Tews DS, Goebel HH, Meinck HM. DNA-fragmentation and apoptosis-related proteins of muscle cells in motor neuron disorders. Acta Neurol Scand. 1997;96:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Smith HK, Maxwell L, Martyn JA, Bass JJ. Nuclear DNA fragmentation and morphological alterations in adult rabbit skeletal muscle after short-term immobilization. Cell Tissue Res. 2000;302:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Podhorska-Okolow M, Sandri M, Zampieri S, Brun B, Rossini K, Carraro U. Apoptosis of myofibres and satellite cells: exercise-induced damage in skeletal muscle of the mouse. Neuropathol Appl Neurobiol. 1998;24:518-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Borisov AB, Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec. 2000;258:305-318. [PubMed] [DOI] [Full Text] |
| 26. | Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997;273:C579-C587. [PubMed] |
| 27. | Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2005;288:C338-C349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Dupont-Versteegden EE, Fluckey JD, Knox M, Gaddy D, Peterson CA. Effect of flywheel-based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J Appl Physiol (1985). 2006;101:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Fischer U, Jänicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 784] [Cited by in RCA: 769] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 30. | Garrido C, Kroemer G. Life's smile, death's grin: vital functions of apoptosis-executing proteins. Curr Opin Cell Biol. 2004;16:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1288-R1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1015-R1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Siu PM, Alway SE. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol. 2005;565:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Alway SE, Degens H, Krishnamurthy G, Chaudhrai A. Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2003;58:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Vital functions for lethal caspases. Oncogene. 2005;24:5137-5148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 36. | Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115-123. [PubMed] |
| 37. | Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol (1985). 2006;100:1770-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Ruetten H, Badorff C, Ihling C, Zeiher AM, Dimmeler S. Inhibition of caspase-3 improves contractile recovery of stunned myocardium, independent of apoptosis-inhibitory effects. J Am Coll Cardiol. 2001;38:2063-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1730-R1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Prunet C, Lemaire-Ewing S, Ménétrier F, Néel D, Lizard G. Activation of caspase-3-dependent and -independent pathways during 7-ketocholesterol- and 7beta-hydroxycholesterol-induced cell death: a morphological and biochemical study. J Biochem Mol Toxicol. 2005;19:311-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Hamada M, Sumi T, Iwai S, Nakazawa M, Yura Y. Induction of endonuclease G-mediated apopotosis in human oral squamous cell carcinoma cells by protein kinase C inhibitor safingol. Apoptosis. 2006;11:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 368] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 44. | Siu PM, Alway SE. Deficiency of the Bax gene attenuates denervation-induced apoptosis. Apoptosis. 2006;11:967-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |