Published online Dec 14, 2006. doi: 10.3748/wjg.v12.i46.7421
Revised: August 16, 2006
Accepted: August 22, 2006
Published online: December 14, 2006
Use of alcohol is a worldwide habit regardless of socio-economic background. Heavy alcohol consumption is a potential risk factor for induction of pancreatitis. The current review cites the updated literature on the alcohol metabolism, its effects on gastrointestinal and pancreatic function and in causing pancreatic injury, genetic predisposition of alcohol induced pancreatitis. Reports describing prospective mechanisms of action of alcohol activating the signal transduction pathways, induction of oxidative stress parameters through the development of animal models are being presented.
- Citation: Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: An overview. World J Gastroenterol 2006; 12(46): 7421-7427
- URL: https://www.wjgnet.com/1007-9327/full/v12/i46/7421.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i46.7421
Alcohol abuse and dependence are the major cause of morbidity and mortality in the United States. About three-fourths of individuals of 18-26 years of age and two-thirds of those 26 and older are current drinkers. The age related pattern for concurrent alcohol and tobacco dependence was similar to that found for tobacco dependence[1,2]. In a recent survey it was found that approximately 17.6 million adult Americans abuse alcohol or is alcoholic. Alcohol related problems cost society approximately $185 billion per year. In a study conducted at the population level, mortality from pancreatitis due to alcohol addiction was reported[3]. The mechanism of induction of alcoholic pancreatitis is not well understood.
Pancreatitis due to alcohol abuse is a very painful and potentially fatal condition. About one-third of acute pancreatitis cases in the United States are alcohol induced and 60%-90% of pancreatitis patients have a history of chronic alcohol consumption. It is estimated that drinking more than 80 gm of alcohol/d or about 10-11 standard U.S. drinks for a minimum of 6-12 years is required to produce symptomatic pancreatitis[4]. The risk of developing the disease increases with both amount and duration of alcohol consumption. Only 5% of clinically documented alcoholics develop disease but at autopsy only 5%-10% of alcoholics are found to have evidence of chronic pancreatitis[5-7]. Chronic pancreatitis is mostly caused by heavy alcohol consumption and is characterized by onset of symptoms in the 4th or 5th decade[8]. Due to this discrepancy in data of alcoholic and diseased patients, it is thought that other factors like environmental, genetic, race and concomitant risk factors are also involved.
Cigarette smoking might have an additive effect with alcohol in inducing pancreatitis. In the rat model of alcohol-induced pancreatitis, ethanol induces pancreatic ischemia while cigarette smoke potentiates the impairment of pancreatic capillary perfusion caused by ethanol[9]. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis[10,11]. A dietary component may also interact and modify effects of alcohol on the pancreas. A protein and fat rich diet along with continued consumption of alcohol exacerbate the course of chronic pancreatitis[12,13]. African Americans are affected more than Caucasians and this could be due to differences in diet, type or quantity of alcohol consumption[14]. It can be due to differences in metabolism of alcohol in liver and pancreas. Alcohol consumption at intoxicating concentrations induces pancreatic cellular injury that may involve class III isoenzymes of ADH[15].
It is well known that alcohol is metabolized via an oxidative and a non-oxidative pathway in the liver. Various studies have been conducted to demonstrate alcohol metabolism in isolated pancreatic acini and cultured acinar cells. Haber et al[15] have studied oxidative metabolism of alcohol using cultured pancreatic cells. His findings corroborate a study reported by Gukovskaya et al[16], conduced with isolated pancreatic acini. In the cytosol of acini, ethanol is oxidized to acetaldehyde by alcohol dehydrogenase. At physiological blood alcohol concentrations, cytochrome P-450 accounts for approximately 20% of ethanol metabolism[17,18]. The presence of cytochrome P-450 CYP2E1 has been demonstrated in rat pancreas[19] as well as human pancreas[20]. Chronic use of alcohol has been shown to induce the expression of CYP2E1 in rat pancreas[19] similar to that in liver[21].
The non-oxidative pathway of ethanol metabolism involves the formation of FAEE using FAEE synthase[22]. Gukovskaya et al[16] have confirmed the presence of FAEE synthase activity in pancreas that may explain the accumulation of FAEE in pancreas after ethanol exposure. Werner et al[23,24] studied correlation between oxidative and non-oxidative pathways of ethanol metabolism in the pancreas. Their studies show that ethanol causes a dose-dependent injury to pancreas due to a shift to non-oxidative metabolism following inhibition of the oxidative pathway. This results in an increase of FAEE. Carboxyl ester lipase (CEL) has been known to catalyze FAEE synthesis from fatty acids and ethanol. CEL gene polymorphism, especially an increase in the frequency of the L-allele, was found to be associated with alcohol induced pancreatitits[25]. The aspect that inhibition of the oxidative pathway can cause an increase in flux of ethanol metabolism via the non-oxidative pathway needs to be clarified further.
In alcoholic pancreatitis, mutations of the cationic trypsinogen gene and of pancreatic secretory trypsin inhibitor (SPINK-1) have been implicated in its pathogenesis[26]. The discovery of genetic cause of hereditary pancreatitis has renewed interest in genetic predisposition to alcoholic pancreatitis. Various gene mutations like cationic trypsinogen (especially at codon 29 and 1220), SPINK1 mutation, and CFTR gene mutation are associated with development of chronic pancreatitis[27]. In idiopathic pancreatitis, both variants of SPINK1 (N 291 and R 122H) and CFTR were identified. Alteration of both genes was found in patients with alcoholic chronic pancreatitis and the increase of those genes is related to higher levels of alcohol consumption[27]. The SPINK1 inhibits the autoactivation of premature trypsinogen within the pancreas[28]. The most frequent mutation in the SPINK1 gene was the N34S mutation in Exon-3. Kuwata et al[29] detected intronic polymorphism in the SPINK1 gene in 4 alcoholic patients but found no differences when the data were compared with other patients. Similar findings have been reported in five other alcoholic chronic pancreatitis patients with an N34S mutation[30]. Truninger et al[31] and Monaghan et al[32] screened 58 and 46 alcoholic pancreatitis patients respectively for cationic trypsinogen gene mutation activation but no mutations have been found.
Alcohol metabolizing enzymes such as aldehyde dehydrogenase (ADH), metabolizing alcohol to acetaldehyde, exist as different isoenzymes. ADH polymor-phism has been identified in ADH-2 and ADH-3[33]. Increased prevalence of ADH-1 isoenzyme in patients with alcoholic pancreatitis has been reported[34]. With regard to ADH-2 isoenzyme, results are not conclusive and further studies need to be done. Kimura et al[35] and Frenzer et al[36] found no correlation between ADH-2 polymorphism and chronic pancreatitis in Japanese and Austrian patients respectively while Maruyama et al[37] demonstrated elevated risk for chronic alcoholic pancreatitis for different genotypes of ADH-2. Cytochrome P-450 is involved in metabolizing alcohol in endoplasmic reticulum. Several studies have been done to evaluate the association between polymorphism of enzyme Cyto-P4502E1 (CYP2E1) and alcoholic pancreatitis with no success[36,38,39]. Verlaan et al[40] analyzed DNA samples from chronic pancreatitis -alcoholic, idiopathic and hereditary. They observed that the frequency of ADH-3 and CYP2E1C1C2 genotypes did not differ between chronic pancreatitis patients, alcoholic and healthy controls. But they conclude that the presence of CYP2E1 intron 6DD genotype might confer a higher risk of alcoholic chronic pancreatitis. Kim et al[41] compared the genotype and allele frequencies of ADH-2, ADH-3, ALDH-2, Cyto P450-2E1, IL-1, IL-6, IL-8 and TNF-alpha in patients with chronic pancreatitis and alcoholic liver cirrhosis with those from controls. No difference in frequencies of genotype and allele of enzymes and cytokines amongst three groups were found. Frequency of ADH-2 was significantly higher and those of CYP2E1 and ALDH-2 were significantly lower than control.
Polymorphism of other enzymes involved in free radical stress and acinar cell damage, such as glutathione -S-transferase (GST) family has also been investigated[42]. Bartsch et al[43] found a moderate increase in susceptibility of pancreatitis due to polymorphism of GSTM1 but Frenzer et al[36] and Scheider et al[44] were not able to confirm it. The enzymes GSTM1, GSTT1, GSTP1, CYP1A1 and CYP2E1 are involved in bioactivation and detoxification of a variety of xenobiotics present in smoke, alcoholic drink among others. A higher frequency of the Val/Val genotype in alcoholics and pancreatitis in comparison to alcoholics without the disease was found[45]. In the same study the investigators found an association between the occurrence of Val/Val GSTP1 genotype and chronic pancreatitis and also an association between m2/m2, CYP1A1 and alcoholic liver cirrhosis suggesting that these genotypes are genetically more prone to the development of alcoholic pancreatitis and alcoholic cirrhosis respectively.
Ethanol had a stimulatory effect on gastric acid secre-tion[46]. Singer et al[47] found that the effect of ethanol on gastric acid secretion is concentration dependent. Gastric instillation of 1.4% and 4.0% (v/v) of ethanol has a small stimulatory effect while higher concentrations (up to 40% v/v) have either no effect or an inhibitory one. Different studies under controlled conditions have been conducted to determine the action of pure ethanol on gastric acid secretion but results were very different from each other[48-54]. Alcohol is oxidized by ADH enzyme present in all parts of the gut[55,56]. Ethanol has a direct toxic effect on the mucosal epithelium leading to loss of epithelium and hemorrhagic erosions in the duodenum[57,58]. In large bowel, gut flora plays an important role in ethanol metabolism[59]. Due to increased amounts of conversion of ethanol to acetaldehyde, than to further oxidize to acetate, the toxic acetaldehyde accumulates thereby damaging colonic mucosa and after being absorbed into the portal blood may contribute to liver injury. Alcohol when taken orally is known to increase mucosal perfusion[60] and also to stimulate production of secretin[61]. Both can affect pancreas microcirculation indirectly. Ethanol is also known to affect pancreatic blood flow when given intravenously[62,63] and via intragastric route[64]. McKim et al[65] investigated the effect of chronic intragastric ethanol administration which induced pancreatic hypoxia and oxidative stress in vivo. Pancreatic hypoxia induced by chronic alcohol appears to be secondary to increase in oxygen consumption by pancreas or to decrease in local blood supply without alteration of hemodynamic patterns. Chronic ethanol ingestion was associated with dose related inhibition of basal pancreatic protein secretion which was reversed upon alcohol withdrawal[66]. Increased susceptibility to chronic alcoholic pancreatitis may be through a hyperstimulation mechanism due to combination of neurohormonal factors. In exocrine pancreas, alcohol induces secretory alteration which varies by manner and duration of alcohol exposure. Ethanol effects on pancreatic secretion appear to be primarily caused by systemic cholinergic mechanisms of the vagus nerve[67].
Various studies have been done to understand the mechanism of ethanol induced pancreatic injury but till now the exact mechanism is not clear. Earlier it was thought that the Sphincter of Oddi spasm induced by alcohol may be one of the mechanisms responsible but due to a lack of consensus, the later proposal includes the Ductal-Plug hypothesis by Sarles and his colleagues[68]. The secretion of pancreatic juice rich in protein may “plug” the small ductules leading to acinar atrophy and fibrosis. It was not clear whether protein precipitation within pancreatic ducts precedes acinar damage. So protein plugs may be a cause or an effect of pancreatic injury. Saluja and Bhagat[69] investigated the mechanism by which alcohol may induce pancreatitis in an animal model. Ethanol administration resulted in a transient increase of pancreatic amylase output and plasma cholecystokinin (CCK) levels. This phenomenon was mediated by a trypsin-sensitive CCK-releasing factor from the duodenum. The studies suggest that ethanol-induced stimulation of pancreatic digestive enzyme secretion plays an important role in the development of pancreatic injury. Chronic ethanol exposure alone in animals inhibits apoptosis through an intrinsic pathway and the downstream apoptosis executor caspase-3 when compared with the controls[70]. Alcohol exposure accelerates pancreatic necrosis in response to endotoxin. The results from this study showed that the pancreas exposed to alcohol is more sensitive to LPS-induced damage because of increased sensitivity to necrotic cell death rather than apoptotic cell death suggesting that this mechanism may occur in acute alcoholic pancreatitis patients[70].
Due to inability to explain the pathogenesis of alcoholic pancreatitis by theories as mentioned above the focus was directed to pancreatic acinar cells. It is now believed that acinar cells are capable of metabolizing alcohol and the toxic effect may predispose the gland to injury in the presence of an appropriate triggering factor. The characteristics of pancreatic stellate cells showing the involvement of acinar cells in pancreatic fibrosis may be another possible link[71] . It is speculated that metabolites of ethanol like acetaldehyde and FAEEs have direct effects on acinar cells/or induce metabolic alterations within cells indirectly. Acetaldehyde is believed to interfere with the binding of secretagogue to their receptors[72] and thereby inhibit stimulated secretion from isolated pancreatic acini[72]. It also causes microtubule dysfunction thereby affecting exocytosis from acinar cells[73].
During oxidation of ethanol, hydrogen ions and reducing equivalents are released[74]; increase NADH, thereby leading to an imbalance between free radicals and antioxidant defense mechanism. It leads to a loss of mitochondrial glutathione and inactivation of GPx and other respiratory complexes[75]. Also, chronic ethanol ingestion upregulates CYP2E1[19] and catalase[76] for metabolism These pathways will require increased oxygen that will compete with mitochondrial electron transport system leading to localized and transient hypoxia in tissues. These transient conditions of hypoxia and re-oxygenation would further enhance ROS formation through the respiratory chain.
FAEEs, products of non-oxidative ethanol metabolism, have been shown to induce pancreatic injury in vivo[77] and in vitro[78]. FAEE undergo hydrolysis to FFA which impairs mitochondrial function by uncoupling of mitochondrial and oxidative phosphorylation[79]. Also its direct binding to the intracellular membrane leads to alteration in function and permeability of cell membrane[80]. The generation of cholestryl esters is responsible for the increase in lysosomal fragility releasing hydrolase’s which act on the zymogen granule membrane and increase release of trypsin[81,82].
Impairment of blood flow to pancreas by ethanol causes hypoxia without any change in hemodynamic parameters. McCord[83] explained reoxygenation induced injury following hypoxia. Hypoxia can decrease the ability of cells to detoxify free radicals[84] and secondarily, hypoxia/reoxygenation causes more free radical formation leading to formation of α-hydroxyethyl radical and subsequent tissue damage and functional impairment. McKim et al[65] found 4-hydroxy nonenal protein adduct accumulation and increasing hypoxia in the pancreas following chronic intragastric alcohol administration in rats. These studies support the hypothesis that hypoxia contributes to oxidative stress caused by alcohol.
The mechanism of acute and chronic ethanol mediated pancreatic injury is unclear in the literature. Feeding alcohol to animals could not reproduce pancreatitis, suggesting that alcohol alone is not sufficient to induce pancreatitis. It sensitizes pancreas to other risk factors, thereby injuring pancreas[85]. Studies using CCK or its analog are done to induce pancreatitis both in vivo and in vitro[86]. CCK at supra-physiological doses causes pancreatitis with increased blood levels of amylase and lipase and acute inflammatory response along with parenchymal cell damage[87-89]. Katz et al[90] showed that ethanol with low dose CCK-8 caused 6-fold more zymogen conversion than that caused by CCK alone. To evaluate a mechanism for the development of alcoholic pancreatitis, Pandolet al[91] fed animals intragastrically with ethanol diet followed by infusion of CCK-8. Ethanol exposure in the presence of CCK-8 resulted in activation of pro-inflammatory transcription factors, NF-κB, AP-1 and other cytokine and inflammatory molecules thereby resulting in increased trypsin release. On the other hand alcohol when given alone causes less activation of NF-κB as compared to that given with CCK alone indicating that ethanol has inhibitory effects on the inflammatory response alone. Ca2+ and PKC contribute to NF-κB activation induced by CCK-8 in acinar cells. Ethanol differentially affects the Ca (2+)/calcineurin-and PKC-mediated pathways of NF-kappaB activation in pancreatic acinar cells[16,92]. These effects may play a role to sensitize pancreas to the inflammatory response and pancreatitis. Acute oxidative stress modulates secretion and repetitive Ca2+ spiking in rat pancreas[93]. Thus perturbations in Ca2+ signaling do not fully explain the secretory block caused by oxidative stress in acute pancreatitis.
Cigarette smoking is a known risk factor for alcoholic and chronic pancreatitis. About 80%-95% of people who abuse alcohol also smoke while 25%-30% of smokers do not drink alcohol[94]. The incidence of alcoholism is 10 times more likely in smokers than nonsmokers. Cigarette smoking accelerates the progression of alcohol induced pancreatitis[10,11]. Blomqvist et al[95] reported that intermittent nicotine administration to rats enhances ethanol intake and preference to ethanol in a free choice between ethanol and water. He suggested that subchronic nicotine doses increased the responsiveness of mesolimbic dopamine neurons to both nicotine and alcohol. Potthoff et al[96] found similar results in their experiments in rats administering chronic nicotine. Ericson et al[97] reported the involvement of nicotinic acetylcholine receptors (nAChR) in nicotine induced increased uptake of ethanol. He gave antagonist to peripheral nAChR to mice and rats subchronically for 15 d and after stopping drug, ethanol intake and preference as well as ethanol induced locomotor stimulation increased. This may be due to compensatory enhanced autonomic ganglionic and/or muscarinic neurotransmission. The mechanism (hormonal or metabolic) by which increased peripheral neuronal activity affects the brain dopaminergic system in the brain is not known.
The mechanism by which alcohol causes the pancreatic injury is not entirely clear. Metabolic as well as microcirculatory changes and other theories were proposed to explain this phenomenon. Cigarette smoking is known as a potentiating factor in the development of alcohol induced pancreatic injury. Hartwig et al[9] investigated the effect of cigarette smoke on alcohol induced pancreatic injury. They gave cigarette smoke alone or with ethanol intravenously to rats. Ethanol alone impairs pancreatic blood flow without any change in systemic hemodynamic parameters and inflammatory change. Cigarette smoke potentiates pancreatic microcirculatory impairment by ethanol and also induces leukocyte aggregation and adhesion.
Nicotine is metabolized by cytochrome P450 into cotinine[98,99]. Tissue distribution of 3H-nicotine in rats demonstrated that nicotine is distributed and accumulated significantly in the pancreas and parts of the gastrointestinal tract[100]. But nicotine metabolism in pancreas has not been reported yet. In liver, low doses of nicotine and ethanol induces CYP2E1 activity as reported by Howard et al[101]. The study suggests that nicotine may increase CYP2E1-induced toxicity and contribute to cross-tolerance in smokers and people treated with nicotine. It may be possible that nicotine might have some effect on pancreatic CYP2E1 induction leading to increased metabolism of ethanol in pancreas by cytochrome and thereby potentiate the damage caused by ethanol.
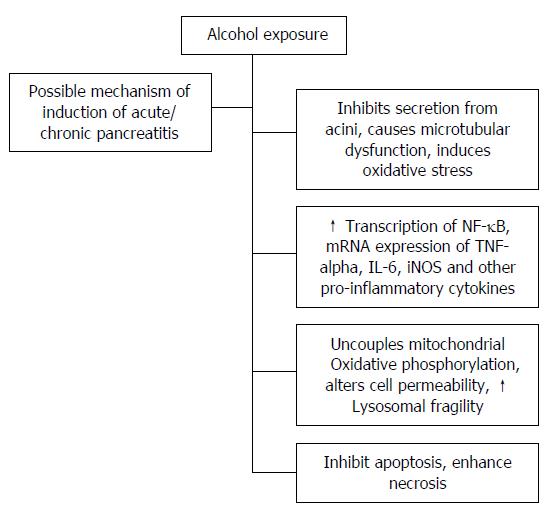
Alcohol abuse/alcoholism are a major cause of pancreatitis. Combining alcohol abuse with smoking aggravates the condition further. Despite numerous reported studies the pathogenesis of alcoholic pancreatitis remains obscure. During recent years it has been possible to evaluate the mechanism of development of alcoholic pancreatitis in the animal model and in in-vitro acinar cell cultures. The summary of events relating to alcohol exposure that may lead to induction of alcoholic pancreatitis is shown in the flow diagram below (Figure 1).
| 1. | Substance abuse and Mental Health Services Administration. Summary of findings from the 2000 National Household Survey on Drug Abuse. . |
| 2. | Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Res Health. 2000;24:201-208. [PubMed] |
| 3. | Ramstedt M. Alcohol and pancreatitis mortality at the population level: experiences from 14 western countries. Addiction. 2004;99:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Strate T, Yekebas E, Knoefel WT, Bloechle C, Izbicki JR. Pathogenesis and the natural course of chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Schenker S, Montalvo R. Alcohol and the pancreas. Recent Dev Alcohol. 1998;14:41-65. [PubMed] |
| 6. | Ammann RW. The natural history of alcoholic chronic pancreatitis. Intern Med. 2001;40:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 7. | Strate T, Knoefel WT, Yekebas E, Izbicki JR. Chronic pancreatitis: etiology, pathogenesis, diagnosis, and treatment. Int J Colorectal Dis. 2003;18:97-106. [PubMed] |
| 8. | Otte M. Chronic pancreatis and pancreatic carcinoma in the elderly. Schweiz Rundsch Med Prax. 2005;94:943-948. |
| 9. | Hartwig W, Werner J, Ryschich E, Mayer H, Schmidt J, Gebhard MM, Herfarth C, Klar E. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Maisonneuve P, Lowenfels AB, Müllhaupt B, Cavallini G, Lankisch PG, Andersen JR, Dimagno EP, Andrén-Sandberg A, Domellöf L, Frulloni L. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Apte MV, Pirola RC, Wilson JS. Where there's smoke there's not necessarily fire. Gut. 2005;54:446-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Pitchumoni CS. Pathogenesis of alcohol-induced chronic pancreatitis: facts, perceptions, and misperceptions. Surg Clin North Am. 2001;81:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Lankisch MR, Imoto M, Layer P, DiMagno EP. The effect of small amounts of alcohol on the clinical course of chronic pancreatitis. Mayo Clin Proc. 2001;76:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Lowenfels AB, Maisonneuve P, Grover H, Gerber E, Korsten MA, Antunes MT, Marques A, Pitchumoni CS. Racial factors and the risk of chronic pancreatitis. Am J Gastroenterol. 1999;94:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Haber PS, Apte MV, Applegate TL, Norton ID, Korsten MA, Pirola RC, Wilson JS. Metabolism of ethanol by rat pancreatic acinar cells. J Lab Clin Med. 1998;132:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Gukovskaya AS, Mouria M, Gukovsky I, Reyes CN, Kasho VN, Faller LD, Pandol SJ. Ethanol metabolism and transcription factor activation in pancreatic acinar cells in rats. Gastroenterology. 2002;122:106-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517-544. [PubMed] |
| 18. | Matsumoto H, Matsubayashi K, Fukui Y. Evidence that cytochrome P-4502E1 contributes to ethanol elimination at low doses: effects of diallyl sulfide and 4-methyl pyrazole on ethanol elimination in the perfused rat liver. Alcohol Clin Exp Res. 1996;20:12A-16A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Norton ID, Apte MV, Haber PS, McCaughan GW, Pirola RC, Wilson JS. Cytochrome P4502E1 is present in rat pancreas and is induced by chronic ethanol administration. Gut. 1998;42:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Foster JR, Idle JR, Hardwick JP, Bars R, Scott P, Braganza JM. Induction of drug-metabolizing enzymes in human pancreatic cancer and chronic pancreatitis. J Pathol. 1993;169:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Johansson I, Ekström G, Scholte B, Puzycki D, Jörnvall H, Ingelman-Sundberg M. Ethanol-, fasting-, and acetone-inducible cytochromes P-450 in rat liver: regulation and characteristics of enzymes belonging to the IIB and IIE gene subfamilies. Biochemistry. 1988;27:1925-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 247] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Lange LG. Nonoxidative ethanol metabolism: formation of fatty acid ethyl esters by cholesterol esterase. Proc Natl Acad Sci USA. 1982;79:3954-3957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Werner J, Saghir M, Warshaw AL, Lewandrowski KB, Laposata M, Iozzo RV, Carter EA, Schatz RJ, Fernández-Del Castillo C. Alcoholic pancreatitis in rats: injury from nonoxidative metabolites of ethanol. Am J Physiol Gastrointest Liver Physiol. 2002;283:G65-G73. [PubMed] |
| 24. | Werner J, Saghir M, Fernandez-del Castillo C, Warshaw AL, Laposata M. Linkage of oxidative and nonoxidative ethanol metabolism in the pancreas and toxicity of nonoxidative ethanol metabolites for pancreatic acinar cells. Surgery. 2001;129:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Miyasaka K, Ohta M, Takano S, Hayashi H, Higuchi S, Maruyama K, Tando Y, Nakamura T, Takata Y, Funakoshi A. Carboxylester lipase gene polymorphism as a risk of alcohol-induced pancreatitis. Pancreas. 2005;30:e87-e91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Gullo L, Migliori M, Brunetti MA, Manca M. Alcoholic pancreatitis: new insights into an old disease. Curr Gastroenterol Rep. 2005;7:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Keim V. [Chronic pancreatitis--pancreas cancer: influence of genetic factors]. Praxis (Bern 1994). 2005;94:811-817. [PubMed] |
| 28. | Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 667] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 29. | Kuwata K, Hirota M, Sugita H, Kai M, Hayashi N, Nakamura M, Matsuura T, Adachi N, Nishimori I, Ogawa M. Genetic mutations in exons 3 and 4 of the pancreatic secretory trypsin inhibitor in patients with pancreatitis. J Gastroenterol. 2001;36:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Drenth JP, te Morsche R, Jansen JB. Mutations in serine protease inhibitor Kazal type 1 are strongly associated with chronic pancreatitis. Gut. 2002;50:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Truninger K, Malik N, Ammann RW, Muellhaupt B, Seifert B, Müller HJ, Blum HE. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. Am J Gastroenterol. 2001;96:2657-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Monaghan KG, Jackson CE, KuKuruga DL, Feldman GL. Mutation analysis of the cystic fibrosis and cationic trypsinogen genes in patients with alcohol-related pancreatitis. Am J Med Genet. 2000;94:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Bosron WF, Li TK. Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology. 1986;6:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 391] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 34. | Haber P, Wilson J, Apte M, Korsten M, Pirola R. Individual susceptibility to alcoholic pancreatitis: still an enigma. J Lab Clin Med. 1995;125:305-312. [PubMed] |
| 35. | Kimura S, Okabayashi Y, Inushima K, Kochi T, Yutsudo Y, Kasuga M. Alcohol and aldehyde dehydrogenase polymorphisms in Japanese patients with alcohol-induced chronic pancreatitis. Dig Dis Sci. 2000;45:2013-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Frenzer A, Butler WJ, Norton ID, Wilson JS, Apte MV, Pirola RC, Ryan P, Roberts-Thomson IC. Polymorphism in alcohol-metabolizing enzymes, glutathione S-transferases and apolipoprotein E and susceptibility to alcohol-induced cirrhosis and chronic pancreatitis. J Gastroenterol Hepatol. 2002;17:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Maruyama K, Takahashi H, Matsushita S, Nakano M, Harada H, Otsuki M, Ogawa M, Suda K, Baba T, Honma T. Genotypes of alcohol-metabolizing enzymes in relation to alcoholic chronic pancreatitis in Japan. Alcohol Clin Exp Res. 1999;23:85S-91S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Yang B, O'Reilly DA, Demaine AG, Kingsnorth AN. Study of polymorphisms in the CYP2E1 gene in patients with alcoholic pancreatitis. Alcohol. 2001;23:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Chao YC, Young TH, Chang WK, Tang HS, Hsu CT. An investigation of whether polymorphisms of cytochrome P4502E1 are genetic markers of susceptibility to alcoholic end-stage organ damage in a Chinese population. Hepatology. 1995;22:1409-1414. [PubMed] |
| 40. | Verlaan M, Te Morsche RH, Roelofs HM, Laheij RJ, Jansen JB, Peters WH, Drenth JP. Genetic polymorphisms in alcohol-metabolizing enzymes and chronic pancreatitis. Alcohol Alcohol. 2004;39:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Kim MS, Lee DH, Kang HS, Park HS, Jung S, Lee JW, Kwon KS, Kim PS, Kim HG, Shin YW. [Genetic polymorphisms of alcohol-metabolizing enzymes and cytokines in patients with alcohol induced pancreatitis and alcoholic liver cirrhosis]. Korean J Gastroenterol. 2004;43:355-363. [PubMed] |
| 42. | Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1476] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 43. | Bartsch H, Malaveille C, Lowenfels AB, Maisonneuve P, Hautefeuille A, Boyle P. Genetic polymorphism of N-acetyltransferases, glutathione S-transferase M1 and NAD(P)H: quinone oxidoreductase in relation to malignant and benign pancreatic disease risk. The International Pancreatic Disease Study Group. Eur J Cancer Prev. 1998;7:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Schneider A, Tögel S, Barmada MM, Whitcomb DC. Genetic analysis of the glutathione s-transferase genes MGST1, GSTM3, GSTT1, and GSTM1 in patients with hereditary pancreatitis. J Gastroenterol. 2004;39:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Burim RV, Canalle R, Martinelli Ade L, Takahashi CS. Polymorphisms in glutathione S-transferases GSTM1, GSTT1 and GSTP1 and cytochromes P450 CYP2E1 and CYP1A1 and susceptibility to cirrhosis or pancreatitis in alcoholics. Mutagenesis. 2004;19:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Beazell JM, Ivy AC. The influence of alcohol on the digestive tract, a review. J Stud Alcohol. 1940;1:45-60. |
| 47. | Singer MV, Leffmann C, Eysselein VE, Calden H, Goebell H. Action of ethanol and some alcoholic beverages on gastric acid secretion and release of gastrin in humans. Gastroenterology. 1987;93:1247-1254. [PubMed] |
| 48. | McArthur K, Hogan D, Isenberg JI. Relative stimulatory effects of commonly ingested beverages on gastric acid secretion in humans. Gastroenterology. 1982;83:199-203. [PubMed] |
| 49. | Singer MV, Eysselein V, Goebell H. Beer and wine but not whisky and pure ethanol do stimulate release of gastrin in humans. Digestion. 1983;26:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Peterson WL, Barnett C, Walsh JH. Effect of intragastric infusions of ethanol and wine on serum gastrin concentration and gastric acid secretion. Gastroenterology. 1986;91:1390-1395. [PubMed] |
| 51. | Singer MV, Leffmann C. Alcohol and gastric acid secretion in humans: a short review. Scand J Gastroenterol Suppl. 1988;146:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Singer MV, Teyssen S, Eysselein VE. Action of beer and its ingredients on gastric acid secretion and release of gastrin in humans. Gastroenterology. 1991;101:935-942. [PubMed] |
| 53. | Teyssen S, González-Calero G, Schimiczek M, Singer MV. Maleic acid and succinic acid in fermented alcoholic beverages are the stimulants of gastric acid secretion. J Clin Invest. 1999;103:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Teyssen S, Lenzing T, González-Calero G, Korn A, Riepl RL, Singer MV. Alcoholic beverages produced by alcoholic fermentation but not by distillation are powerful stimulants of gastric acid secretion in humans. Gut. 1997;40:49-56. [PubMed] |
| 55. | Pestalozzi DM, Bühler R, von Wartburg JP, Hess M. Immunohistochemical localization of alcohol dehydrogenase in the human gastrointestinal tract. Gastroenterology. 1983;85:1011-1016. [PubMed] |
| 56. | Seitz HK, Egerer G, Oneta C, Krämer S, Sieg A, Klee F, Simanowski UA. Alcohol dehydrogenase in the human colon and rectum. Digestion. 1996;57:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Barona E, Pirola RC, Leiber CS. Small intestinal damage and changes in cell population produced by ethanol ingestion in the rat. Gastroenterology. 1974;66:226-234. [PubMed] |
| 58. | Kelly JP, Kaufman DW, Koff RS, Laszlo A, Wiholm BE, Shapiro S. Alcohol consumption and the risk of major upper gastrointestinal bleeding. Am J Gastroenterol. 1995;90:1058-1064. [PubMed] |
| 59. | Salaspuro M. Bacteriocolonic pathway for ethanol oxidation: characteristics and implications. Ann Med. 1996;28:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Beck IT. The role of splanchnic circulation and mucosal microcirculatory changes in ethanol-induced small bowel injury. Pathophysiology of the Splanchnic Circulation. Boca Raton, FL: CRC Press 1978; 197-201. |
| 61. | Straus E, Urbach HJ, Yalow RS. Alcohol-stimulated secretion of immunoreactive secretin. N Engl J Med. 1975;293:1031-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Horwitz LD, Myers JH. Ethanol-induced alterations in pancreatic blood flow in conscious dogs. Circ Res. 1982;50:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Foitzik T, Fernández-del Castillo C, Rattner DW, Klar E, Warshaw AL. Alcohol selectively impairs oxygenation of the pancreas. Arch Surg. 1995;130:357-360; discussion 361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Widdison AL, Alvarez C, Schwarz M, Reber HA. The influence of ethanol on pancreatic blood flow in cats with chronic pancreatitis. Surgery. 1992;112:202-208; discussion 208-210. [PubMed] |
| 65. | McKim SE, Uesugi T, Raleigh JA, McClain CJ, Arteel GE. Chronic intragastric alcohol exposure causes hypoxia and oxidative stress in the rat pancreas. Arch Biochem Biophys. 2003;417:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Deng X, Wood PG, Eagon PK, Whitcomb DC. Chronic alcohol-induced alterations in the pancreatic secretory control mechanisms. Dig Dis Sci. 2004;49:805-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Siegmund SV, Singer MV. [Effects of alcohol on the upper gastrointestinal tract and the pancreas--an up-to-date overview]. Z Gastroenterol. 2005;43:723-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Sarles H. Alcoholism and pancreatitis. Scand J Gastroenterol. 1971;6:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Saluja AK, Bhagat L. Pathophysiology of alcohol-induced pancreatic injury. Pancreas. 2003;27:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Fortunato F, Deng X, Gates LK, McClain CJ, Bimmler D, Graf R, Whitcomb DC. Pancreatic response to endotoxin after chronic alcohol exposure: switch from apoptosis to necrosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G232-G241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Apte MV, Pirola RC, Wilson JS. Molecular mechanisms of alcoholic pancreatitis. Dig Dis. 2005;23:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Sankaran H, Lewin MB, Wong A, Deveney CW, Wendland MF, Leimgruber RM, Geokas MC. Irreversible inhibition by acetaldehyde of cholecystokinin-induced amylase secretion from isolated rat pancreatic acini. Biochem Pharmacol. 1985;34:2859-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Ponnappa BC, Hoek JB, Waring AJ, Rubin E. Effect of ethanol on amylase secretion and cellular calcium homeostasis in pancreatic acini from normal and ethanol-fed rats. Biochem Pharmacol. 1987;36:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Lieber CS. Metabolism of ethanol and associated hepatotoxicity. Drug Alcohol Rev. 1991;10:175-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 391] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 76. | Bradford BU, Enomoto N, Ikejima K, Rose ML, Bojes HK, Forman DT, Thurman RG. Peroxisomes are involved in the swift increase in alcohol metabolism. J Pharmacol Exp Ther. 1999;288:254-259. [PubMed] |
| 77. | Werner J, Laposata M, Fernández-del Castillo C, Saghir M, Iozzo RV, Lewandrowski KB, Warshaw AL. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Haber PS, Wilson JS, Apte MV, Pirola RC. Fatty acid ethyl esters increase rat pancreatic lysosomal fragility. J Lab Clin Med. 1993;121:759-764. [PubMed] |
| 79. | Lange LG, Sobel BE. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J Clin Invest. 1983;72:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 184] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Hungund BL, Goldstein DB, Villegas F, Cooper TB. Formation of fatty acid ethyl esters during chronic ethanol treatment in mice. Biochem Pharmacol. 1988;37:3001-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Wilson JS, Colley PW, Sosula L, Pirola RC, Chapman BA, Somer JB. Alcohol causes a fatty pancreas. A rat model of ethanol-induced pancreatic steatosis. Alcohol Clin Exp Res. 1982;6:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Wilson JS, Apte MV, Thomas MC, Haber PS, Pirola RC. Effects of ethanol, acetaldehyde and cholesteryl esters on pancreatic lysosomes. Gut. 1992;33:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3970] [Cited by in RCA: 3779] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 84. | Jones DP. The role of oxygen concentration in oxidative stress: Hypoxic and hyperoxic models. Oxidative Stress. London: Academic Press 1985; 151-195. |
| 85. | Pandol SJ, Gukovsky I, Satoh A, Lugea A, Gukovskaya AS. Emerging concepts for the mechanism of alcoholic pancreatitis from experimental models. J Gastroenterol. 2003;38:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Pandol SJ, Gukovsky I, Satoh A, Lugea A, Gukovskaya AS. Animal and in vitro models of alcoholic pancreatitis: role of cholecystokinin. Pancreas. 2003;27:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Gorelick FS, Adler G, Kern HF. Cerulein-induced pancreatitis. The Pancreas: Biology, Pathobiology, and Disease. New York: Raven 1993; 501-526. |
| 88. | Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 408] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 89. | Sandoval D, Gukovskaya A, Reavey P, Gukovsky S, Sisk A, Braquet P, Pandol SJ, Poucell-Hatton S. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology. 1996;111:1081-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 90. | Katz M, Carangelo R, Miller LJ, Gorelick F. Effect of ethanol on cholecystokinin-stimulated zymogen conversion in pancreatic acinar cells. Am J Physiol. 1996;270:G171-G175. [PubMed] |
| 91. | Pandol SJ, Periskic S, Gukovsky I, Zaninovic V, Jung Y, Zong Y, Solomon TE, Gukovskaya AS, Tsukamoto H. Ethanol diet increases the sensitivity of rats to pancreatitis induced by cholecystokinin octapeptide. Gastroenterology. 1999;117:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 92. | Gukovskaya AS, Hosseini S, Satoh A, Cheng JH, Nam KJ, Gukovsky I, Pandol SJ. Ethanol differentially regulates NF-kappaB activation in pancreatic acinar cells through calcium and protein kinase C pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G204-G213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Sweiry JH, Shibuya I, Asada N, Niwa K, Doolabh K, Habara Y, Kanno T, Mann GE. Acute oxidative stress modulates secretion and repetitive Ca2+ spiking in rat exocrine pancreas. Biochim Biophys Acta. 1999;1454:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Batel P, Pessione F, Maître C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 179] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 95. | Blomqvist O, Ericson M, Johnson DH, Engel JA, Söderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 197] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 96. | Potthoff AD, Ellison G, Nelson L. Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav. 1983;18:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Ericson M, Engel JA, Söderpalm B. Peripheral involvement in nicotine-induced enhancement of ethanol intake. Alcohol. 2000;21:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Hammond DK, Bjercke RJ, Langone JJ, Strobel HW. Metabolism of nicotine by rat liver cytochromes P-450. Assessment utilizing monoclonal antibodies to nicotine and cotinine. Drug Metab Dispos. 1991;19:804-808. [PubMed] |
| 99. | Nakayama H, Okuda H, Nakashima T, Imaoka S, Funae Y. Nicotine metabolism by rat hepatic cytochrome P450s. Biochem Pharmacol. 1993;45:2554-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Chowdhury P, Doi R, Chang LW, Rayford PL. Tissue distribution of [3H]-nicotine in rats. Biomed Environ Sci. 1993;6:59-64. [PubMed] |
| 101. | Howard LA, Micu AL, Sellers EM, Tyndale RF. Low doses of nicotine and ethanol induce CYP2E1 and chlorzoxazone metabolism in rat liver. J Pharmacol Exp Ther. 2001;299:542-550. [PubMed] |