Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.644
Revised: July 28, 2005
Accepted: August 11, 2005
Published online: January 28, 2006
AIM: To explore the role of transforming growth factor-beta1 (TGF-β1)-smad signal transduction pathway in patients with hepatocellular carcinoma.
METHODS: Thirty-six hepatocellular carcinoma specimens were obtained from Qidong Liver Cancer Institute and Department of Pathology of the Second Affiliated Hospital of Nanjing Medical University. All primary antibodies (polyclonal antibodies) to TGF-β1, type II Transforming growth factor-beta receptor (TβR-II), nuclear factor-kappaB (NF-κB), CD34, smad4 and smad7,secondary antibodies and immunohistochemical kit were purchased from Zhongshan Biotechnology Limited Company (Beijing, China). The expressions of TGF-β1, TβR-II, NF-κB, smad4 and smad7 proteins in 36 specimens of hepatocellular carcinoma (HCC) and its adjacent tissue were separately detected by immunohistochemistry to observe the relationship between TGF-β1 and TβR-II, between NF-κB and TGF-β1, between smad4 and smad7 and between TGF-β1 or TβR-IIand microvessel density (MVD). MVD was determined by labelling the vessel endothelial cells with CD34.
RESULTS: The expression of TGF-β1, smad7 and MVD was higher in HCC tissue than in adjacent HCC tissue (P<0.01, P <0.05, P <0.01 respectively). The expression of TβR-IIand smad4 was lower in HCC tissue than in its adjacent tissue (P <0.01, P <0.05 respectively). The expression of TGF-β1 protein and NF-κB protein was consistent in HCC tissue. The expression of TGF-β1 and MVD was also consistent in HCC tissue. The expression of TβR-IIwas negatively correlated with that of MVD in HCC tissue.
CONCLUSION: The expressions of TGF-β1, TβR-II, NF-κB, smad4 and smad7 in HCC tissue, which are major up and down stream factors of TGF-β1-smad signal transduction pathway , are abnormal. These factors are closely related with MVD and may play an important role in HCC angiogenesis. The inhibitory action of TGF-β1 is weakened in hepatic carcinoma cells because of abnormality of TGF-β1 receptors (such as TβR-II) and postreceptors (such as smad4 and smad7). NF-κB may cause activation and production of TGF-β1.
- Citation: Ji GZ, Wang XH, Miao L, Liu Z, Zhang P, Zhang FM, Yang JB. Role of transforming growth factor-beta1-smad signal transduction pathway in patients with hepatocellular carcinoma. World J Gastroenterol 2006; 12(4): 644-648
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.644
Hepatocellular carcinoma is one of the most common malignant tumors in the world. Recent studies have demonstrated that the key factor of invasiveness and metastasis of HCC is neovascularization. There are two kinds of factors in adjusting neovascularization, one is positive adjusting factors, such as vascular endothelial cell growth factor (VEGF), hepatocellular growth factor(HGF), transforming growth factor alpha (TGF-α), epidermal growth factor (EGF). The other kind includes negative adjusting factors, such as transforming growth factor beta (TGF-β). Synthesis of TGF is adjusted by nucleus transcription factors, such as NF-κB. This study was designed to investigate the significance and mechanism of TGF-β1-smad signal transduction pathway in hepatocellular carcinoma (HCC).
Thirty-six HCC specimens were obtained from Qidong Liver Cancer Institute and Department of Pathology of the Second Affiliated Hospital of Nanjing Medical University. All primary antibodies (polyclonal antibodies) to TGF-β1, TβR-II, NF-κB, CD34, smad4 and smad7 and secondary antibodies were purchased from Zhongshan Biotechnology Limited Company (Beijing, China).
Each specimen was cut into 4-μm thick sections. Tissue wax sections were unfolded on glass sheet. Immunohistochemistry (strepolin-biotin-peroxidase method) was used to detect the expression of TGF-β1,TβR-II, NF-κB, CD34, smad4 and smad7. Briefly, paraffin-embedded tissue sections were dewaxed, treated with 3%H2O2 at 37 °C, washed with PBS, incubated with TGF-β1,TβR-II, NF-κB, CD34, smad4 and smad7 antibodies separately, washed with PBS for 15 min, incubated again with strepolin-biotin -peroxidase at 37 °C. Finally, the sections were washed with PBS for 15 min, visualized with DAB reagent and counterstained with hematoxylin.Negative and positive controls were used simultaneously to ensure specificity and reliability of the staining process.The negative controls were incubated with PBS instead of primary antibody and a positive section supplied by the manufacturer of the staining kit was taken as positive control.Sections were observed under microscope after being mounted.All positive sections were analyzed with RY2000 analysis system. Microvessel density (MVD) was measured as previously described [1]. High vessel density was found in 100× sights.Microvessels in five regions were counted in 400× sights,the average of microvessels with CD34 staining in five regions was calculated as MVD.
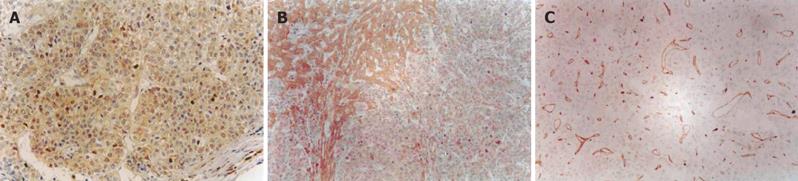
The buffy staining of cell membrane, plasma or nuclei was considered positive staining of TGF-β1,TβR-II, NF-κB,smad4 and smad7 (Figures 1A and 1B). Positive staining of CD34 was considered as microvessels (Figure1C).
The data were expressed as mean ± SD. Chi-square test and t test were used. P<0.05 was considered statistically significant.Kappa value was regarded as consistency degree.
Different expressions of TGF-β1, TβR-II were observed in HCC and its adjacent tissue. There was a significant difference in the positive expressions of TGF-β1, TβR-II between HCC and its adjacent tissue (Table 1).
The positive expression rate of both TGF-β1 and TβR-II was 27.78% (10/36) and their negative expression rate was 11.11% (4/36). The total rate was 38.89% (14/36). The discrepancy between expressions of TGF-β1 and TβR-II was 61.11% (22/36). The consistency degree in statistical test was weak (kappa = 0.25) (Table 2).
| TGF-β1 | Total | |||
| + | − | |||
| + | 10 | 8 | 18 | |
| TβR-II | ||||
| − | 14 | 4 | 18 | |
| Total | 24 | 12 | 36 | |
The MVD was higher in HCC tissue than in its adjacent tissue. There was a significant difference in MVD between HCC and its adjacent tissue (P<0.01, Table 3).
| n | MVD | |
| HCC tissue | 36 | 32.45 ± 10.62 |
| Adjacent tissue | 36 | 4.62 ± 1.67 |
The MVD in HCC tissue was higher in positive expression group of TGF-β1 than in negative expression group of TGF-β1. The difference was significant between the two groups (P<0.05). The MVD in HCC tissue in positive expression group of TβR-IIwas lower than in negative expression group of TβR-II. The difference was significant between the two groups (P<0.01)
Among the 36 specimens, 19 (52.78%) had positive expression of TGF-β1 and NF-κB in HCC tissue. The negative expression rate of TGF-β1 and NF-κB was 22.22% (8/36)in HCC tissue. The consistent rate of TGF-β1 and NF-κB was 75.00% (27/36).
The positive expression rate of smad4 in HCC and its adjacent tissue was 19.44% (7/36) and 75.00% (21/28) respectively. There was a significant difference between them (P<0.01). The positive expression rate of smad7 in HCC tissue and its adjacent tissue was 63.89% (23/36) and 35.71% (10/28) respectively. There was a significant difference between them (P<0.05).
Tumor growth, invasion, metastasis depend on angiogenesis. Through its vessels, tumor can obtain rich nutrients and secrete tumor cells, resulting in tumor growth and metastasis. MVD of tumor is a valid marker to reflect tumor angiogenesis. Chen et al[2] reported that HCC MVD can be reflected by dynamic enhancement of spiral CT scanning. MVD of tumor has been detected by marking vessel endothelium. Vascular endothelial growth factor (VEGF) is an important angiogenic factor regulating tumor angiogenesis. Yao et al[3] reported that the VEGF expression rate is 63.9% in HCC, 78.3% in non-encapsulated HCC, and 90.9% in HCC with extrahepatic metastasis, respectively. The VEGF expression is closely correlated with MVD. Moon et al[4] reported that overproduction of VEGF and angiopoietin 2 in HCC cells may increase vascularity and tumor growth in a paracrine manner. Poon et al[5] reported that the MVD in HCC tissue is higher than that in its adjacent tissue. The MVD in HCC tissue is associated with hepatocarcinoma prognosis. The higher the MVD is, the poorer the prognosis is. Our study revealed the same result, suggesting that tumor angiogenesis plays an important role in tumor genesis and development.
TGF-β is an important adjusting factor and has five isoforms: TGF-β1-5. TGF-β1-3 have the same biological functions with 70-80% homology. TGF-β1 is a negative adjusting factor in tumor growth. There are three kinds of TGF-β receptors: TβR-I, TβR-II, TβR-III. Their relative molecules are 65×103,85-110×103 and 6×105 respectively. TβR-I and TβR-II playing a part in signal transduction are serine/ threonine protein kinase receptors with a single span membrane. The section of extracellular membrane is short, while the section of cell plasma is long. The section of extracellular membrane contains rich cysteine, the section of cell plasma contains serine/ threonine protein kinase structure and possess its activity. Compared to TβR-II, the out and inner sections of cellular membrane of TβR-I are short. TGF-β1 combines with TβR-II becoming dimer, then with TβR-I becoming trimer. The activated TβR-I and phosphorate-acidulated smad could adjust transcription of genes. TGF-β1 is over-expressed in tumor tissue, but tumor cells do not respond to suppressive factor TGF-β1, suggesting that the signal transduction pathway is abnormal. TGF-β plays an important role as a ligand in tumor genesis suppressing cell growth in early stage and promoting cell growth in advanced stage. TGF-β1 exerts its strong suppressive effect on hepatocellular proliferation. Kim et al[6] showed that TGF-β1 could induce hepatocyte apoptosis. Abou-Shady et al[7] detected TGF-β mRNA in normal and HCC tissue with Northern blot method and found that TGF-β mRNA is over-expressed in HCC and its adjacent tissue. Idobe et al[8] compared the intensity of TGF-β1 staining in HCC and its adjacent tissue and found that the former is more intense than the latter. The expression of TGF-β1 in HCC tissue is correlated with the histological differentiation, namely the lower the histological differentiation of HCC, the more intense the TGF-β1 staining in HCC tissue. Matsuzaki et al[9] showed that TGF-β1 mRNA is over-expressed in HCC cells and TGF-β1 accelerates their proliferation. Giannelli et al[10] reported that invasive HCC cells express alpha3 beta1-integrin whereas noninvasive HCC cells do not. TGF-β1 stimulates alpha3-integrin expression at a transcriptional level in noninvasive HCC cells, causing transformation into a motile and invasive phenotype. Invasive HCC cells secrete abundant active TGF-β1 in comparison to noninvasive HCC cells. Anti-TGF-β1 neutralizing antibody reduces alpha3-integrin expression and the ability of HCC to invade cells, suggesting that TGF-β1 may play an important role in HCC invasiveness by stimulating alpha3-integrin expression, and may be an important target for new therapies. Cai et al[11] showed that TGF-β1 treatment can enhance the amount of alpha5 beta 1-integrin on HCC cell surface, the mRNA level of alpha5 subunit, subsequently stimulating cell adhesion to fibronectin (Fn) and laminin (Ln) matrix. TGF-β1 can also promote cell migration. Song et al[12] reported that TGF-β1 may be a useful serologic marker in detecting HCC at its initial stage because it shows higher sensitivity and specificity in the diagnosis of small HCC. Ueno et al[13] reported that expression of TβR-II in liver tissues is significantly decreased in patients with HCC compared to that in patients with chronic hepatitis or liver cirrhosis. They transfected T beta RII cDNA to hepatoma cell line (Huh7) and compared the change of cell number and observed the induction of apoptosis after TGF-beta1 treatment using a FACScan flow cytometer. In Huh7 cells transfected with T beta RII cDNA, cell arrest and apoptosis were obviously induced. We have previously shown that expression of TGF-β1 mRNA and protein in HCC tissue is higher than that in its adjacent tissue[14]. Our study displayed that expression of TGF-β1 was enhanced, and expression of TβR-II was weak in HCC tissue compared to that in its adjacent tissue, which may be due to the lower expression of TβR-II in HCC cells[13] that can escape from the inhibitory effects of TGF-β1, thus causing genesis and development of HCC.
Smad is an essential signal transducer of TGF-beta signal pathway. At present, ten kinds of smad have been found: smad1-10. Smad4 is an action substrate of TGF-β receptor and has been identified as a tumor suppressor gene. Mutation or lower expression of smad4 has been observed in many kinds of tumor[15-18]. In normal hepatic tissue, smad4 plays an essential role in signal transduction of TGF-β and influences gene transcription, controls cell growth and guides the suppressive role of TGF-β in hepatic cell growth. Tannapfel et al[19] reported that expression of smad4 is decreased in HCC tissue. Our study showed that expression of smad4 was lower in HCC tissue than in its adjacent tissue, suggesting that expression of smad4 is abnormal in HCC tissue, resulting in blocking signal transduction of TGF-β1 and taking part in hepatic carcinoma cell escaping from the suppression of TGF-β1. smad7 is an inhibitor of smad and can bind to TβR-I, blocking phosphorylation of smad and signal transduction of TGF-β1. It is thus considered as an oncogene. Kawate et al [20] investigated mutation of smad7 in HCC tissue using polymerase chain reaction-single strand conformation polymorphism analysis and found that smad7 displays single nucleotide polymorphisms. Our study showed that expression of smad7 was higher in HCC tissue than in its adjacent tissue, suggesting that smad7 may take part in hepatic carcinoma cell escaping from suppression of TGF-β1.
Synthesis of TGF-β1 is controlled by nuclear transcription factors. At present, the main nuclear transcription factors are nuclear factor-kappaB (NF-κB) and activator protein 1. NF-κB could bind to enhancer κB of immunoglobulin kappa light chain gene. NF-κB is a dimer of p65 and p50. In normal conditions, NF-κB exists in cell plasma. Inhibitory factor of NF-κB (I-κB) falls off NF-κB complex after stimulated. Activated NF-κB enters into nuclei and accelerates transcription of target gene. Because tissue transglutaminase gene promotor (a TGF-β1 activated factor) contains binding sites of NF-κB, NF-κB can accelerate expression of TGF-β1. It was reported that hepatic carcinoma cells could excrete TGF-β1[21]. Our study showed that expression of NF-κB was closely related with TGF-β1 expression.
In conclusion, TGF-β1, TβR-II,NF-κB, smad4 and smad7 in HCC tissue are the major factors in TGF-β1-smad signal transduction pathway. These factors are closely related with MVD and may play an important role in HCC angiogenesis. NF-κB may cause the activation and production of TGF-β1.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Liu WF
| 1. | Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9-19. [PubMed] |
| 2. | Chen WX, Min PQ, Song B, Xiao BL, Liu Y, Ge YH. Single-level dynamic spiral CT of hepatocellular carcinoma: correlation between imaging features and density of tumor microvessels. World J Gastroenterol. 2004;10:67-72. [PubMed] |
| 3. | Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, Wu W, Qiu LW, Meng XY. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:220-226. [PubMed] |
| 4. | Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma. Mod Pathol. 2003;16:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Kim BC, Mamura M, Choi KS, Calabretta B, Kim SJ. Transforming growth factor beta 1 induces apoptosis through cleavage of BAD in a Smad3-dependent mechanism in FaO hepatoma cells. Mol Cell Biol. 2002;22:1369-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Abou-Shady M, Baer HU, Friess H, Berberat P, Zimmermann A, Graber H, Gold LI, Korc M, Büchler MW. Transforming growth factor betas and their signaling receptors in human hepatocellular carcinoma. Am J Surg. 1999;177:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Idobe Y, Murawaki Y, Kitamura Y, Kawasaki H. Expression of transforming growth factor-beta 1 in hepatocellular carcinoma in comparison with the non-tumor tissue. Hepatogastroenterology. 2003;50:54-59. [PubMed] |
| 9. | Matsuzaki K, Date M, Furukawa F, Tahashi Y, Matsushita M, Sakitani K, Yamashiki N, Seki T, Saito H, Nishizawa M. Autocrine stimulatory mechanism by transforming growth factor beta in human hepatocellular carcinoma. Cancer Res. 2000;60:1394-1402. [PubMed] |
| 10. | Giannelli G, Fransvea E, Marinosci F, Bergamini C, Colucci S, Schiraldi O, Antonaci S. Transforming growth factor-beta1 triggers hepatocellular carcinoma invasiveness via alpha3beta1 integrin. Am J Pathol. 2002;161:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Cai T, Lei QY, Wang LY, Zha XL. TGF-beta 1 modulated the expression of alpha 5 beta 1 integrin and integrin-mediated signaling in human hepatocarcinoma cells. Biochem Biophys Res Commun. 2000;274:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Song BC, Chung YH, Kim JA, Choi WB, Suh DD, Pyo SI, Shin JW, Lee HC, Lee YS, Suh DJ. Transforming growth factor-beta1 as a useful serologic marker of small hepatocellular carcinoma. Cancer. 2002;94:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Ueno T, Hashimoto O, Kimura R, Torimura T, Kawaguchi T, Nakamura T, Sakata R, Koga H, Sata M. Relation of type II transforming growth factor-beta receptor to hepatic fibrosis and hepatocellular carcinoma. Int J Oncol. 2001;18:49-55. [PubMed] |
| 14. | Ji GZ, Zhao ZQ, Miu L, Liu Z, Zhang P, Yang C, Huang J. Studies on expressions of TGF-β1mRNA and protein in patients with hepatocellular carcinoma. Jiangsu Yiyao Zazhi. 2003;29:485-487. |
| 15. | de Vos tot Nederveen Cappel WH, Lagendijk MA, Lamers CB, Morreau H, Vasen HF. Surveillance for familial pancreatic cancer. Scand J Gastroenterol Suppl. 2003;94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Kuroda H, Matsunaga T, Terui T, Tanaka I, Takimoto R, Fujikawa K, Takayama T, Kato J, Hirayama Y, Sakamaki S. Decrease of Smad4 gene expression in patients with essential thrombocythaemia may cause an escape from suppression of megakaryopoiesis by transforming growth factor-beta1. Br J Haematol. 2004;124:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Stancu M, Wu TT, Wallace C, Houlihan PS, Hamilton SR, Rashid A. Genetic alterations in goblet cell carcinoids of the vermiform appendix and comparison with gastrointestinal carcinoid tumors. Mod Pathol. 2003;16:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Tang ZH, Zou SQ, Hao YH, Wang BJ, Yang XP, Chen QQ, Qiu FZ. The relationship between loss expression of DPC4/Smad4 gene and carcinogenesis of pancreatobiliary carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:624-629. [PubMed] |
| 19. | Tannapfel A, Anhalt K, Häusermann P, Sommerer F, Benicke M, Uhlmann D, Witzigmann H, Hauss J, Wittekind C. Identification of novel proteins associated with hepatocellular carcinomas using protein microarrays. J Pathol. 2003;201:238-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Kawate S, Ohwada S, Hamada K, Koyama T, Takenoshita S, Morishita Y, Hagiwara K. Mutational analysis of the Smad6 and Smad7 genes in hepatocellular carcinoma. Int J Mol Med. 2001;8:49-52. [PubMed] |
| 21. | Sugano Y, Matsuzaki K, Tahashi Y, Furukawa F, Mori S, Yamagata H, Yoshida K, Matsushita M, Nishizawa M, Fujisawa J. Distortion of autocrine transforming growth factor beta signal accelerates malignant potential by enhancing cell growth as well as PAI-1 and VEGF production in human hepatocellular carcinoma cells. Oncogene. 2003;22:2309-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |