Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.608
Revised: July 28, 2005
Accepted: August 26, 2005
Published online: January 28, 2006
AIM: To determine the feasibility of performing computed tomography (CT)-guided transpulmonary radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) located in the hepatic dome.
METHODS: A total of seven patients with HCC comprising seven nodules located in the hepatic dome were treated from April 2004 to December 2004. CT-guided transpulmonary RFA was performed using a cool-tip type electrode (Radionics Company) based on a standardized energy protocol. All tumors located in the hepatic dome were not detectable by the usual ultrasound (US) methods. The lesion diameters ranged from 15 to 27 mm.
RESULTS: RFA was technically feasible in all the patients. The puncture procedure was performed twice or less and the total average performance time was 40.6 min. Local tumor control was achieved in all the patients. The necrosis diameter ranged from 25 to 35 mm. The mean follow-up period was 9.6 (7-14 mo) mo. There was no local recurrence at the follow-up points. Pneumothorax requiring pleural drainage was the main complication, which was observed in two of the seven patients (28.6%). However, it improved with chest drainage tube, and the tube could be removed within 2-3 d. No other major complications were observed.
CONCLUSION: CT-guided puncture is useful for the treatment of tumors located in the hepatic dome which are hardly detectable by US, even though pneumothorax sometimes may occur as a complication. In the cases with adhesion in the pleura for which artificial pleural effusion methods are not appropriate, CT-guided RFA is thus considered to be an alternative treatment for HCC located in the hepatic dome.
- Citation: Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, Takagi H, Mori M, Nakajima T. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol 2006; 12(4): 608-611
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/608.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.608
Percutaneous radiofrequency ablation (RFA) is a standard therapy for hepatocellular carcinoma (HCC)[1-4]. Ultrasound (US)-guided puncture is the simplest and most popular method for performing RFA treatment of HCC[1-4]. However, depending on the location of tumors, it is often difficult to detect them by US[5-7]. Especially, the subphrenic area is one of the most difficult places because the lung obstructs the ultrasound[6,7]. As a result, some approaches using artificial pleural effusion method[6-8], thoracoscopy[9,10] or open surgery have been performed for HCC located in the hepatic dome. However, each approach has its advantages and disadvantages. The artificial pleural effusion method is sometimes complicated and requires a high level of skill for the performer. Furthermore, patients with adhesion of the pleura are not indicated for this procedure. A thoracoscopic approach or open surgery needs general anesthesia. Recently, RFA has been performed for the treatment of various neoplasms including lung cancer[11,12]. Although pneumothorax is a major complication observed in 16% of all cases, lung RFA has shown itself to be a safe and feasible option for the treatment of lung neoplasms[11]. Shibata et al[13] have previously reported that CT-guided transpulmonary RFA is effective for eight patients with liver tumors in the hepatic dome. We have herein determined the utility of CT-guided transpulmonary RFA for the treatment of HCC located in the subphrenic area. The aim of this study was to assess the feasibility and efficacy of CT-guided transpulmonary RFA for HCC.
A total of seven patients (five males, two females, mean age 67.9 ± 5.9 years) with HCC comprising seven nodules not indicative of undergoing usual US-guided RFA were included in this study and treated from April 2004 to December 2004. In four of these patients, transcatheter arterial embolization (TAE) for HCC was performed previously. In two patients, US-guided RFA was initially attempted but proved to be impossible to perform. The patients’ characteristics are given in Table 1. RFA was performed after an interdisciplinary consensus conference, with the participation of hepatologists, radiologists, and surgeons. After detailed patient information was given, written consent was obtained. The inclusion criteria for RFA were a rejection of surgical therapy by the surgeon or the patient. The exclusion criteria were tumor size of 3 cm or more than three liver lesions, a prothrombin time < 50%, and thrombocytes < 50 000/μL. Tumors detectable with usual US methods were excluded. Within 2 wk prior to RFA, all patients underwent preinterventional CT imaging.
| Patients | Age/Sex | Preinterventional therapy | Child-Pugh classification | Number of tumor treated with trans-pulmonary RFA | Size (mm) | Location | Puncture procedure (times) | Puncture procedure (min) | Complication | Follow-up (mo) |
| 1 | 60/M | TAE | A | 1 | 20 | S8 | 2 | 50 | Pneumothorax need the chest drainage tube | 12 |
| 2 | 71/M | TAE | A | 1 | 15 | S8 | 2 | 36 | Pneumothorax need the chest drainage tube | 9 |
| 3 | 77/F | TAE | A | 1 | 16 | S8 | 1 | 30 | No | 14 |
| 4 | 63/M | RFA | A | 1 | 27 | S8/4 | 2 | 55 | Small pneumothorax | 10 |
| 5 | 68/F | RFA | A | 1 | 15 | S8 | 1 | 34 | Small pneumothorax | 8 |
| 6 | 72/M | no | A | 1 | 15 | S8 | 1 | 40 | Small pneumothorax | 7 |
| 7 | 64/M | TAE | A | 1 | 22 | S8 | 2 | 39 | No | 7 |
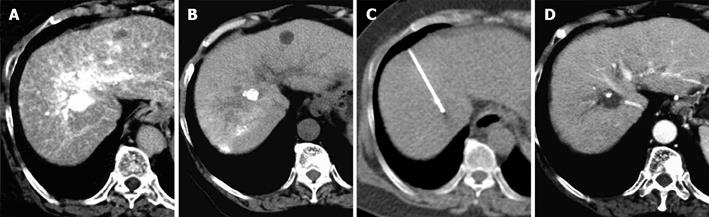
After local anesthesia, a 17-gauge cooled-tip electrode with a 2- or 3 cm exposed tip (Radionics, Burlington, MA, USA) was attached to a radiofrequency generator and then inserted under CT guidance. The patient was placed in a supine position and two neutral electrodes were placed at the proximal thigh. For the localization of lesions, the skin entrance point was validated with a grid made from sealed plastic tubes. Thereafter, the cool-tip needle was inserted into the lesion. The punctures were controlled with CT imaging, which was acquired using the breath-hold technique. Finally, the position was checked by CT imaging. After successful placement of the RFA needle, RFA was started. Both the temperature and tissue impedance were monitored during ablation. Cooled water was infused to maintain the tip temperature at < 20 °C. The radiofrequency energy was delivered for 6-12 min each time. For large lesions, the electrode was inserted into additional sites of the tumor nodule. After the ablation procedure was finished, CT was acquired with the RFA needle still in place to evaluate the size of necrosis and to rule out any remaining viable tumor parts in the region of ablation. Dynamic CT was then used to determine whether or not the tumor nodule was completely ablated and necrotized just after the procedure and a few days after RFA. For further follow-up, dynamic CT was performed after 1 mo and then every 3 mo after the procedure. Technical success was defined as complete tumor necrosis without any remaining contrast-enhanced tumor tissue a few days and 1 mo after the ablation procedure.
The cool-tip needle was successfully placed and RFA was successfully performed in all the patients. All the patients presented with interventional pain and were treated with a single dose of intravenous anesthesia. No further complications occurred during the intervention. The duration of the entire RFA procedure ranged 34-55 min (mean 40.6 min). The puncture procedure was performed twice or less and the average puncture time was 1.57 ± 0.53 times. The 1-mo follow-up examination showed a complete local tumor ablation in all the patients. The mean follow-up period was 9.6 (range 7-14 mo) mo. There was no local tumor recurrence in any patient. Two patients had intrahepatic recurrences and their treated lesions remained tumor-free. All patients survived 6 mo after the ablation. Five patients remained completely tumor-free during the follow-up period. The results are given in detail in Table 1 and a representative case is shown in Figure 1. Pneumothorax was noted in five cases (71.4%), including two cases (28.6%) of major pneumothorax requiring a chest tube. However, both of these patients improved due to chest drainage and the tube could be removed within 2-3 d. In addition, none of the patients demonstrated any clinical complications with problematic pleural effusion. Trans-pulmonary RFA showed itself to be a safe and feasible option in the treatment of HCC located in the hepatic dome.
CT-guided transpulmonary RFA was successfully performed with a good local control for HCC by means of this relatively easy method in the present study. Percutaneous RFA is a standard therapy for HCC[1-4]. One advantage of such percutaneous techniques is its repeatability in cases of local or distant tumor recurrence and its minimal invasive characteristics in comparison to surgery[1-4]. Furthermore, RFA has been proved to be superior regarding the extent of tumor necrosis in comparison to percutaneous ethanol injection (PEI) for the treatment of small HCC[14]. However, depending on the location of tumors, it is often difficult to detect them by US, especially in the subphrenic area. Minami et al[6] have reported percutaneous US-guided RFA with the concurrent use of artificial pleural effusion for HCC located in the right subphrenic region. After 200-1 100 mL of 50 g/L glucose solution was infused intrathoracically to separate the lung and the liver, it became possible to obtain an image of the whole tumor, suggesting that US-guided RFA with artificial pleural effusion for HCC is safe and has no major complications. Although US-guided RFA with artificial pleural effusion is a good approach with minimal invasion[6-8], its procedure is sometimes complicated and requires the performer with a high degree of experience. The success of US-guided RFA with artificial pleural effusion thus depends on the skill of the performer. In addition, some patients with adhesion of the pleura may not be indicated for artificial pleural effusion. CT-guided transpulmonary RFA does not require any infusion of the artificial pleural effusion and is thus easier to detect the tumor and to insert the needle. Although US allows for real-time surveillance of the puncture procedure, the lesion is sometimes insufficiently visualized and artifacts may hamper precise needle placement. Especially in the case of liver cirrhosis, CT imaging may sometimes be helpful in distinguishing malignant liver nodules from benign regenerative nodules[14,15]. In addition, the CT-guided technique enables us to determine whether the tumor nodule is completely ablated and necrotized just after the procedure by dynamic CT. We avoided any reintervention due to the remaining vital tumor tissue at the site of ablation.
The transpulmonary approach has been proven to be a safe and feasible option in the treatment of lung neoplasms although pneumothorax is a major complication[11,12]. Pneumothorax requiring pleural drainage is the main complication of RFA for lung cancer[11]. Lung RFA has shown itself to be a safe and feasible option for the treatment of lung cancer[11]. In our study, the diaphragm was also punctured with RFA needle when the patient underwent CT-guided trans-pulmonary RFA for HCC. Fortunately, there was no major complication concerning diaphragm injury or massive pleural effusion. Shibata et al[13] also reported that percutaneous transthoracic RFA can be used in the treatment of liver tumors in the hepatic dome. They experienced a case of moderate pleural effusion[13], suggesting that all the patients undergoing this procedure should thus be carefully watched for pleural effusion. In our study, two of seven patients required a chest tube. Fortunately, we did not experience cases complicated by uncontrollable pneumothorax. When the patients have lung emphysema, pneumothorax may be more complicated and uncontrollable. The indication for trans-pulmonary RFA should be restricted for such cases complicated by lung emphysema.
In conclusion, CT-guided trans-pulmonary RFA for patients with HCC in the hepatic dome may be a safe and potentially effective therapy. In the cases for which artificial pleural effusion methods are not appropriate, CT guidance may therefore be a useful modality.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Kong LH
| 1. | Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: Ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127:S159-S166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Qian J, Feng GS, Vogl T. Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol. 2003;9:1885-1891. [PubMed] |
| 3. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 4. | Beaugrand M, N'kontchou G, Seror O, Ganne N, Trinchet JC. Local/regional and systemic treatments of hepatocellular carcinoma. Semin Liver Dis. 2005;25:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 740] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 6. | Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Inoue T, Sakaguchi Y, Sakamoto H, Shiozaki H. Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol. 2003;38:1066-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004;183:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Minami Y, Kudo M, Kawasaki T, Chung H, Ogawa C, Shiozaki H. Percutaneous radiofrequency ablation guided by contrast-enhanced harmonic sonography with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. AJR Am J Roentgenol. 2004;182:1224-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Ishikawa T, Kohno T, Shibayama T, Fukushima Y, Obi S, Teratani T, Shiina S, Shiratori Y, Omata M. Thoracoscopic thermal ablation therapy for hepatocellular carcinoma located beneath the diaphragm. Endoscopy. 2001;33:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Teramoto K, Kawamura T, Takamatsu S, Nakamura N, Kudo A, Noguchi N, Irie T, Arii S. Laparoscopic and thoracoscopic approaches for the treatment of hepatocellular carcinoma. Am J Surg. 2005;189:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Gadaleta C, Catino A, Ranieri G, Armenise F, Colucci G, Lorusso V, Cramarossa A, Fiorentini G, Mattioli V. Radiofrequency thermal ablation of 69 lung neoplasms. J Chemother. 2004;16 Suppl 5:86-89. [PubMed] |
| 12. | Steinke K, Habicht JM, Thomsen S, Soler M, Jacob AL. CT-guided radiofrequency ablation of a pulmonary metastasis followed by surgical resection. Cardiovasc Intervent Radiol. 2002;25:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Shibata T, Shibata T, Maetani Y, Kubo T, Itoh K, Togashi K, Hiraoka M. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol. 2004;15:1323-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Ikeda M, Okada S, Ueno H, Okusaka T, Kuriyama H. Radiofrequency ablation and percutaneous ethanol injection in patients with small hepatocellular carcinoma: a comparative study. Jpn J Clin Oncol. 2001;31:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ward J, Robinson PJ. How to detect hepatocellular carcinoma in cirrhosis. Eur Radiol. 2002;12:2258-2272. [PubMed] |