Published online Aug 21, 2006. doi: 10.3748/wjg.v12.i31.5021
Revised: March 20, 2006
Accepted: March 27, 2006
Published online: August 21, 2006
AIM: Recently it has been reported that granulocyte colony stimulating factor (G-CSF) can induce hypercoagulability in healthy bone marrow donors. It is conceivable that the induction of a prothrombotic state in a recipient of an organ graft with already impaired perfusion might cause further deterioration in the transplanted organ. This study evaluated whether G-CSF treatment worsens liver perfusion following liver transplantation in the rat model.
METHODS: A non-arterialized rat liver transplantation model was employed to evaluate the effect of G-CSF treatment on the liver in a syngeneic and allogeneic strain combination. Study outcomes included survival time and liver damage as investigated by liver enzymes and liver histology. Observation times were 1 d, 1 wk and 12 wk.
RESULTS: Rats treated with G-CSF had increased incidence and severity of biliary damage following liver transplantation. In these animals, hepatocellular necrosis was accentuated in the centrilobular region. These lesions are indicative of impaired perfusion in G-CSF treated animals.
CONCLUSION: G-CSF should be used with caution in recipients of liver transplantation, as treatment might enhance preexisting, undetected perfusion problems and ultimately lead to ischemia induced biliary complications.
- Citation: Dirsch O, Chi H, Ji Y, Gu YL, Broelsch CE, Dahmen U. Administration of granulocyte colony stimulating factor after liver transplantation leads to an increased incidence and severity of ischemic biliary lesions in the rat model. World J Gastroenterol 2006; 12(31): 5021-5027
- URL: https://www.wjgnet.com/1007-9327/full/v12/i31/5021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i31.5021
In 1975 granulocyte colony stimulating factor (G-CSF) was purified from urine[1] and serum[2]. Nowadays G-CSF is in clinical use and is applied routinely for donor conditioning before stem cell transplantation due to its stem cell mobilizing property. It is also used to treat patients who suffer from infections and neutropenia[3,4] and has been used successfully in endotoxin induced septic conditions[5]. Recently G-CSF has been used in immunosuppressed patients, particularly transplant-recipients, suffering from CMV infection and severe neutropenia[6].
Several clinical studies have evaluated the safety of G-CSF for transplant patients with a special focus on acute cellular rejection[6,7]. Acute cellular rejection, however, is only one potential threat to the transplanted organ. Decreased organ perfusion can lead to impaired function and even graft failure[8]. Impaired perfusion can result from ischemia-reperfusion damage or arterial insufficiency secondary to thrombosis at the site of anastomosis.
Recently it was shown that G-CSF can induce hypercoagulability in healthy bone marrow donors[9,10]. A prothrombotic state when a grafted organ is already compromised by impaired perfusion might exacerbate ischemia and further jeopardize the function of the transplanted organ. This study was designed to establish whether G-CSF treatment aggravates impaired liver perfusion following liver transplantation in the rat model.
A non-arterialized rat liver transplantation model was used to evaluate the effect of G-CSF treatment on the liver in a syngeneic and allogeneic strain combination. Outcomes included survival time and liver damage as investigated by liver enzymes in combination with liver histology. Observation time was set to 1 d, 1 wk and 12 wk.
Female Lew and ACI rats were used as donors and male Lewis (LEW, RT1) rats (Charles River Wiga GmbH, Germany), as recipients. The body weight of male rats was within 250-350 g (10-14 wk old), that of female rats were within 200-300 g (11-14 wk old). The animals were housed under standard animal care conditions and fed with rat chow ad libitum before and after operation. All procedures and housing were carried out according to the German Animal Welfare Legislation.
Animals in the treatment group received G-CSF (Neupogen 48TM, AMGEN GmbH, Germany) at a dose of 100 μg/kg daily by subcutaneous injection starting 5 d prior to the operation until postoperative d 21.
Orthotopic liver transplantation was performed according to the technique described by Kamada[11]. Inhalation anesthesia with Isoflurane was used during the operation.
First step of the surgical procedure was a transverse abdominal incision. The liver of the donor was freed from its ligaments. The small-for-size liver graft was generated by 70% liver resection that included removing the left lateral lobe, the left medial lobe and the middle lobe. The weight of the resected liver lobes as well as the graft itself were recorded. The liver graft was perfused through the portal vein with chilled Ringer’s solution. The organ was preserved at 4°C for a maximum of one hour until placed orthotopically in the recipient’s abdomen. The donor suprahepatic vena cava was anastomosed end-to-end with the recipient suprahepatic vena cava using a continuous 7-0 polypropylene suture. The portal vein anastomosis as well as the infrahepatic vena cava anastomosis were performed by pulling the recipient’s vein over a cuff which was secured with a circumferential 6-0 silk suture. Biliary continuity was restored by tying the bile duct over a stent (Klinika Medical GmbH, Germany).
After transplantation animals were placed into clean cages, and were given free access to tap water and rat chow ad libitum. Animals were observed daily. Body weight loss and general condition including activity, jaundice and bleeding from eyes or nose were recorded. Animals with deteriorating general condition indicated by a weight loss of more than 30% were sacrificed and were classified as dying during the observation period.
After sacrifice a full autopsy was performed. Gross findings were documented using a digital camera (Nikon Coolpix 995). Blood was obtained from the infrahepatic vena cava. Tissue samples (liver, bile duct) were fixed in 4% buffered formalin. Paraffin embedding was performed using standard techniques (Tissue Processor TPC15, Medite Inc.). Paraffin sections (4 μm) were stained with Hematoxylin-Eosin (HE) and Elastic von Gieson (EvG).
Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Alkaline phosphatase (AP) and Bilirubin were measured with an Automated Chemical Analyzer (ADVIA 1650, Bayer AG, Germany).
Damage to the liver was assessed histologically using a semiquantitative scoring system (Table 1). The scoring system included assessment of the portal and lobular inflammatory infiltrate, the extent of hepatocellular necrosis and signs of unspecific hepatocellular damage like a fine vacuolar transformation of the cytoplasm of hepatocytes. The number of mitotic figures and of eosinophilic globular structures in the cytoplasm of hepatocytes were counted. The extent of bile duct inflammation, regenerative changes of biliary epithelial cells and the number of ductules was reported.
| Single cell necrosis | 0: No necrotic hepatocyte in 5 HPF (40 X) |
| 1: 1-10 in 5 HPF | |
| 2: > 10 | |
| Confluent necrosis | 0 |
| 1: Small in size and number | |
| 2: Large size and /or large number | |
| Hepatic mitosis | 0: No mitotic hepatocyte in 5 HPF (40 X) |
| 1: 1-10 in 5 HPF | |
| 2: > 10 (extensive) | |
| Bile duct inflammation damage | 0: No inflammation; |
| 1: A minority of the ducts are cuffed and infiltrated by inflammatory cells | |
| 2: More than an occasional duct showing degenerative changes or focal degenerative changes; Most of the ducts infiltrated by inflammatory cells | |
| 3: Most of the ducts showing luminal disruption, most of the ducts infiltrated by inflammatory cells | |
| Ductular proliferation | 0: None |
| 1: Minimal (small proliferation in a minority of portal tracts) | |
| 2: Mild (most portal tracts but not involving the lobular parenchyma) | |
| 3: Moderate (all portal tracts and extending along the fibrous septa) | |
| 4: Severe (extending along the portal tracts and also slightly involving the lobular parenchyma) | |
| 5: Very severe (diffuse proliferation in the lobular parenchyma) | |
| Fibrosis | 0: None |
| 1: Fibrosis slightly extending portal tracts | |
| 2: Fibrosis extending portal tracts with incomplete septa | |
| 3: Fibrosis with complete septa bridging portal to portal tracts | |
| 4: Incomplete (focal) or complete cirrhosis | |
| Activated Kupffer cells | 0: No activated Kupffer cells |
| 1: Activated Kupffer cells | |
| Eosinophilic globuli | 0: No eosinophilic globuli |
| 1: 1%-5% of all hepatocytes | |
| 2: > 5% | |
| Small vacuolar transformation of the cytoplasm | 0: No small vacuolar transformation of the cytoplasm |
| 1: 1%-30% of all the hepatocytes | |
| 2: > 30% |
The liver damage score was calculated as sum of the scores of all parameters, and the bile duct damage score was calculated as sum of bile duct inflammation and ductular proliferation score.
Data analysis and statistical procedures were performed using SPSS 10.0 (SPSS Inc. USA). All graphs were generated using Sigmaplot 7.0 (SPSS Inc. USA).
The survival rate was depicted as Kaplan-Meier curve. Survival rates in different groups were tested using the log rank test. Biochemistry results and damage scores among different groups were tested using the Mann Whitney Rank Sum test.
Normal rats treated with G-CSF (n = 6) for 5 d showed a mean leucocyte count of 22 neutrophilic cells per nl compared to 15 per nL without G-CSF treatment, indicating the biologic activity of human G-CSF in rats. An isolated increase in the blood level of alkaline phophatase was detected in 1 out of 6 animals. ALT, AST and bilirubin were within their normal range.
Histological evaluation revealed no pathologic changes; especially no alteration of the bile ducts or hepatocellular necrosis.
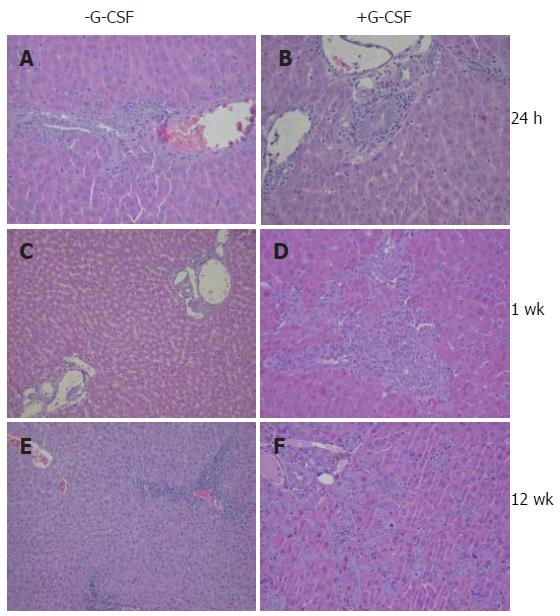
In both the G-CSF treated and untreated groups at 24 h, the liver macroscopically showed slight edema in the hilar region. Histologically a granulocytic infiltrate was found surrounding large hilar intrahepatic bile ducts in 50% of all G-CSF treated rats whereas in untreated rats hardly any inflammatory infiltrates in the large hilar intrahepatic bile ducts was detected (Figure 1).
Alkaline phosphatase was significantly higher in the G-CSF group compared to the untreated group (Table 2) indicating a more pronounced damage to the biliary system in the G-CSF treated animals.
Blood levels of liver enzymes were significantly lower in the G-CSF treated group compared to the untreated group (ALT: G-CSF treated group: 418.67 ± 73.08 μ/L vs untreated group: 634 ± 195.86 μ/L, P = 0.041), indicating a reduced number of damaged liver cells in G-CSF treated animals.
In both animal groups morphologic signs of minor hepatocellular damage like small vacuoles in the cytoplasm and a low number of single cell necrosis were visible.
In the treated group hepatocellular damage was mainly located in the centrilobular region. In contrast this distribution of hepatocytic injury was only observed in 2/6 animals of the untreated group. The other animals presented with single cell necrosis throughout the liver lobule.
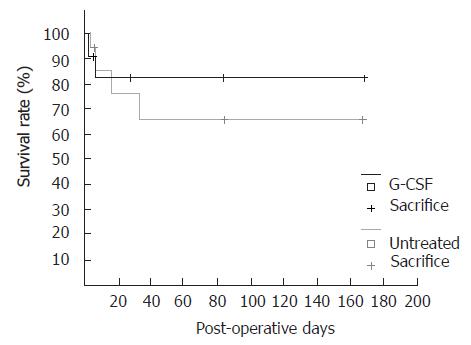
In the one week group with G-CSF treatment all animals survived. Survival rate without G-CSF treatment was 90% (Figure 2).
Upon gross examination, signs of limited inflammation around the common bile duct was found in all animals.
AST and ALT levels in both groups were elevated. Compared to the levels reached 24 h postoperatively, a decrease was observed, indicating recovery (Table 2). Two animals in the treatment group showed a massive increase in the AP-levels indicating severe damage to the bile ducts, whereas the other animals were within the normal range. In the untreated group, three out of six animals showed a slight increase in the AP-levels.
Histologically signs of damage to the biliary epithelial cells were visible in animals with and without G-CSF treatment. After G-CSF treatment rats showed a more pronounced proliferation of small biliary ducts suggesting a bile outflow problem.
Biliary epithelial cells showed signs of regenerative activation indicating repair of cellular damage rats with and without G-CSF treatment. Besides mitotic figures the biliary epithelial cells showed an increased nuclear polymorphism and nuclear enlargement leading to a shift of the nuclear to cytoplasmic ratio. Crowding of the biliary epithelial cells was visible. Nuclei did not show basal orientation. Furthermore signs of ongoing damage to the biliary duct were visible. In the cytoplasm of biliary epithelial cells vacuoles were discernible. A very low number of necrotic biliary epithelial cells could be detected. In the vicinity of bile ducts inflammatory cellular infiltrates were visible.
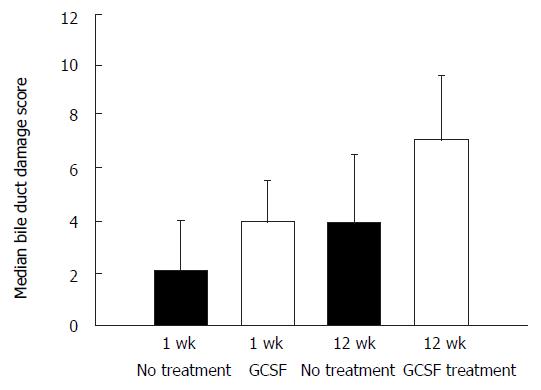
Biliary outflow obstruction is known to induce proliferation of biliary ducts leading to an increase in the number of bile ducts in the portal tracts. Proliferation of bile ducts was more pronounced in animals that underwent G-CSF treatment (Figure 1D). In one rat, the proliferated bile ducts extended into the parenchyma of the liver. In another animal a massive “biliary transformation” of the liver was observed. The biliary duct damage score was slightly higher in G-CSF treated animals at week one, but at this time point the difference did not reach statistical significance (P = 0.074, Mann-Whitney test).
Survival rate at 12 wk post operation was 88% in the untreated group compared to the treated group that showed a survival rate of only 64% (Figure 2).
Macroscopically concrements and sludge in large bile ducts was found slightly more frequent in G-CSF treated rats (3/6 compared to 2/6).
In the untreated group, black sludge in the bile duct was found in one rat. In another rat, a small yellow particle in the common bile duct was found, which was associated with a cystic dilatation of the bile duct.
In G-CSF treated animals a small amount of black sludge was found in 2 out of 6 rats. A 0.4 cm × 0.4 cm black stone-like particle in a dilated bile duct was found in another rat. Totally, macroscopically visible lesions of the large extrahepatic bile duct were found in 3 out of 6 rats.
Blood levels of liver enzymes and AP were increased but comparison of both groups did not show a statistically significant difference at this time point.
Microscopically the number of proliferating small biliary ducts was higher in G-CSF treated animals. The degree of liver cell damage was also higher in G-CSF treated animals. Median bile duct damage score in G-CSF treated animals reached statistical significance compared to rats without G-CSF treatment (Figure 3).
After full size liver transplantation in the ACI to Lewis strain combination animals died within 9 to 14 d. No difference in survival time was observed between animals with and without G-CSF treatment.
Animals dying spontaneously presented with morphological signs of severe rejection, as expected in this strain combination. Scoring of the histopathology according to the Banff-classification showed no difference in severity between the two groups. Portal tracts were wide and filled with activated lymphocytes and other inflammatory cells. Inflammatory cells extended into the parenchyma of the liver and hepatocellular single cell necrosis was obvious.
In addition inflammatory cells infiltrated small bile ducts that showed signs of damage and destruction. Single necrotic biliary cells and vacuolar transformation of biliary epithelium was the characteristic feature. Regenerative activation of biliary epithelial cells was detected. The number of mitotic figures in biliary epithelial cells was increased and nuclei were not evenly arranged in the vicinity of the basal membrane. In addition nuclear enlargement leading to a shift of the nuclear to cytoplasmic ratio was recorded. In addition to ductitis, there was also an inflammatory infiltrate in the luminal parts of the wall of blood vessels both in the portal tract and in the central regions of the lobules. This endothelitis was accompanied by a regenerative activation and proliferation of endothelial cells and in some vessels, minute fibrin deposits were discernible on the luminal surface.
In the G-CSF treated animals the number of granu-locytes seemed to be slightly higher near the bile ducts, whilst hepatocellular single cell necrosis seemed to be slightly more prominent in centrilobular areas compared to rats receiving no G-CSF treatment. Differences were subtle and did not allow for clear discrimination between the two groups. When slides were observed in a blinded fashion animals could not be clearly attributed to either group.
G-CSF is used routinely in conditioning of donors before stem cell transplantations. In donors and healthy volunteers it has been shown that G-CSF induces a prothrombotic state (Table 3). In addition to the increase in prothrombotic factors, enhanced platelet aggregation was also observed, potentially mediated by the G-CSF receptor on platelets[12]. Therefore, it was suspected that G-CSF might lead to thrombotic complications in patients already at risk for thrombosis. Thus, careful monitoring was recommended for patients with additional risk factors for thrombosis[13].
| Author | n | Observations after G-CSF administration or in patients with G-CSF producing tumors |
| Topcuoglu et al, 2004[9] | 18 | Stimulation of thrombotic factors and increased endothelial markers, such as FVIII and vWF |
| No clinical silent microembolism detected by transcranial Doppler | ||
| Sohngen et al, 1998[13] | 25 | Hypercoaguable state |
| LeBlanc et al, 1999[10] | 22 | Increased levels of FVIIIC and thrombin |
| Reduced platelet aggregation. | ||
| Kuroiwa et al, 1996[12] | 10 | Significant increase of platelet aggregation induced by ADP or collagen, thromboxane B2 level and amount of thrombin-antithrombin III complex. |
| Canales et al, 2002[32] | 20 | Significant increase in F1 + 2 and D-dimer |
| Significant decrease of antithrombin and protein C activity | ||
| Significant increase of vWF | ||
| Slightly significant decrease of angiotensin converting enzyme | ||
| Suzuki et al, 1992[33] | 14 | Thrombocytosis |
Furthermore other pathophysiological alterations induced by G-CSF may impair microcirculation. Microcirculation was disturbed in G-CSF treated rats using a free-flap model to investigate behavior of granulocytes after chemotherapy and G-CSF stimulation[14]. Leukocyte rolling and sticking was enhanced to a degree that impaired microcirculation. However, this problem became only visible in this free-flap model with its inherent impairment to microcirculation.
Ischemia-reperfusion injury causing an impairment of microcirculation is unavoidable in transplantations[15-17]. In clinical liver transplantation, impaired arterial perfusion, mostly due to arterial thrombosis in the hepatic artery, is an important cause of graft dysfunction and even failure[18-20]. In the rat, rearterialization is not essential for the survival of the recipient, but is considered by some authors to enhance liver perfusion and especially perfusion of the biliary tract, resulting in a lower rate of biliary complications[21,22]. For this study, a model with a high risk of perfusion related problems was warranted. Therefore, the non-arterialized rat liver transplantation model, as developed by Kamada[20] was employed.
In rats, subjected to liver transplantations and additional perioperative G-CSF treatment, in the early postoperative period we observed a centrilobular accentuation of necrotic hepatocytes. At the same time we also observed an increased blood level of alkaline phosphatase at 24 h. Alkaline phosphatase can be an indicator of damage to the biliary epithelial cells but an increase in the blood levels of alkaline phosphatase after G-CSF treatment is also described in some patients due to the induction of bone metabolism[23]. Especially the pronounced increase after syngeneic liver transplantations in rats treated with G-CSF compared to non-operated G-CSF treated animals suggests damage to the biliary tract leading to cholangitis as the underlying reason.
In the animals observed for 12 wk, an increase in the number and severity of biliary complications and accompanying histomorphological damage was found. In particular, the number of proliferating small biliary ducts, which is indicative of a biliary outflow problem, was more prevalent in G-CSF treated rats.
Impaired arterial perfusion of the liver parenchyma leads to variable centrilobular necrosis[24]. Impaired arterial perfusion of the bile duct leads to damage of the biliary epithelial cells indicated by the release of alkaline phosphatase. Aggressive bile seeping into the surrounding tissue can perpetuate the damage and attract inflammatory cells, causing cholangitis. Especially when larger bile ducts are affected, there can be a functional impairment of smooth muscle cells with loss of motility, which can impair bile outflow. Reduced biliary flow promotes the development of biliary sludge and concrements eventually leading to outflow obstruction. Biliary obstruction induces proliferation of small bile ducts as demonstrated experimentally in a rat liver ischemia model[25] and after bile duct ligation[26].
If this combination of morphological alterations, centrilobular hepatocellular necrosis and cholangitis with or without proliferation of small bile duct is present, as it is in our experiment, an underlying perfusion problem must be assumed. However, it might be difficult to achieve a diagnosis based on histomorphology alone since severe additional confounding alterations like rejection can be superimposed. In the allogeneic strain combination rejection was partially masking perfusion related morphologic alterations. Thus, a perfusion associated problem could only be suspected upon a detailed analysis.
Clinical studies performed to document the safety of G-CSF in transplant patients did not reveal relevant side effects in most studies. G-CSF is known to restore the compromised immune system in neutropenic patients by increasing the number of circulating granulocytes, thus it was suspected that the rejection rate might increase. In the published clinical trials[6,7,27] an influence on rejection rate or severity was not reported, which was also confirmed in our study. Other problems seem to be out of focus in these studies. In a large clinical trial analyzing potential adverse effects of G-CSF in liver transplanted patients[7] not a single case of biliary complications was reported. Biliary problems are rather common after liver transplantations. The complete absence of any biliary problem in such a large cohort of 286 patients is rather surprising.
GCSF-related histomorphological alterations of the liver were reported in studies, where patients were exposed to high levels of circulating cytokines. Suzuki[28] reported on a small number of patients with endocrine tumors producing G-CSF as well as IL-1 and IL-8, who presented with liver dysfunction and fever in addition to marked leukocytosis. Biochemical examinations revealed high serum enzyme levels of the biliary system in contrast to normal or slight increases in transaminase levels in all patients studied. Three common pathologic changes of the liver were found: focal necrosis associated with neutrophil infiltration in the centrilobular zones[1], fibrous change and enlargement of the portal area associated with neutrophil infiltration[2], and intrahepatic cholestasis[3]. The same pathologic changes, except for cholestasis, were observed in the liver of mice transplanted with G-CSF-producing cell lines (KHC287 or CHU-2)[29]. Thus the pattern of liver lesions consisting of an impairment of bile ducts and centrilobular necrosis of hepatocytes was similar to morphologic changes observed in our experiment.
But on the other hand a beneficial effect of G-CSF on the liver after extended liver resection and toxic liver damage has been described[30,31]. Yannaki observed an accelerated recovery and improved survival after liver injury and attributed this effect to the promotion of endogenous repair programs[9], and not to stem cell transdifferentiation, as discussed recently. In the experiment presented here, the lower serum levels of AST and ALT 24 h after liver transplantation could be attributed to this beneficial G-CSF effect. In treated rats, hepatocyte necrosis was only observed in the vicinity of central veins, whereas single cell necrosis was distributed throughout the liver lobule in the untreated group, accompanied by a significantly higher release of liver enzymes. This distribution pattern of single cell necrosis gives rise to the speculation, that GCSF had a beneficial affect on hepatocytes undergoing ischemia reperfusion injury, but had a detrimental effect on perfusion leading to centrilobular necrosis of hepatocytes and necrosis and vacuolization of biliary epithelial cells.
This contradictory effect contributes to the blurring clinical picture, which hinders one from reaching definitive conclusions from these studies. Perfusion related changes are rather subtle, thus it is necessary to give special attention to this problem, which does not always happen, neither in daily clinical routine nor in clinical studies as mentioned before[7].
G-CSF should be used with caution in liver transplanted patients, as treatment might enhance preexisting, undetected perfusion problems and ultimately lead to ischemia induced biliary complications.
S- Editor Wang J L- Editor Worthley DL E- Editor Ma N
| 1. | Kellar KL, Vogler WR, Kinkade JM Jr. Colony stimulating factor (CSF) from human leukemic urine: affinity chromatography and isoelectric focusing. Proc Soc Exp Biol Med. 1975;150:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Metcalf D, MacDonald HR, Chester HM, Metcalf D, MacDonald HR, Chester HM. Serum potentiation of granulocyte and macrophage colony formation in vitro. Exp Hematol. 1975;3:261-273. [PubMed] |
| 3. | García-Carbonero R, Mayordomo JI, Tornamira MV, López-Brea M, Rueda A, Guillem V, Arcediano A, Yubero A, Ribera F, Gómez C. Granulocyte colony-stimulating factor in the treatment of high-risk febrile neutropenia: a multicenter randomized trial. J Natl Cancer Inst. 2001;93:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Schaison G, Eden OB, Henze G, Kamps WA, Locatelli F, Ninane J, Ortega J, Riikonen P, Wagner HP. Recommendations on the use of colony-stimulating factors in children: conclusions of a European panel. Eur J Pediatr. 1998;157:955-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Aoki Y, Hiromatsu K, Kobayashi N, Hotta T, Saito H, Igarashi H, Niho Y, Yoshikai Y. Protective effect of granulocyte colony-stimulating factor against T-cell-meditated lethal shock triggered by superantigens. Blood. 1995;86:1420-1427. [PubMed] |
| 6. | Turgeon N, Hovingh GK, Fishman JA, Basgoz N, Tolkoff-Rubin NE, Doran M, Cosimi AB, Rubin RH. Safety and efficacy of granulocyte colony-stimulating factor in kidney and liver transplant recipients. Transpl Infect Dis. 2000;2:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Winston DJ, Foster PF, Somberg KA, Busuttil RW, Levy MF, Sheiner PA, Reddy KR, Fotheringham N, Armstrong M, Logan E. Randomized, placebo-controlled, double-blind, multicenter trial of efficacy and safety of granulocyte colony-stimulating factor in liver transplant recipients. Transplantation. 1999;68:1298-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Colina F, Mollejo M, Moreno E, Alberti N, García I, Gómez-Sanz R, Castellano G. Effectiveness of histopathological diagnoses in dysfunction of hepatic transplantation. Review of 146 histopathological studies from 53 transplants. Arch Pathol Lab Med. 1991;115:998-1005. [PubMed] |
| 9. | Topcuoglu P, Arat M, Dalva K, Ozcan M. Administration of granulocyte-colony-stimulating factor for allogeneic hematopoietic cell collection may induce the tissue factor-dependent pathway in healthy donors. Bone Marrow Transplant. 2004;33:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | LeBlanc R, Roy J, Demers C, Vu L, Cantin G. A prospective study of G-CSF effects on hemostasis in allogeneic blood stem cell donors. Bone Marrow Transplant. 1999;23:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Kamada N, Calne RY, Wight DG, Lines JG. Orthotopic rat liver transplantation after long-term preservation by continuous perfusion with fluorocarbon emulsion. Transplantation. 1980;30:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Kuroiwa M, Okamura T, Kanaji T, Okamura S, Harada M, Niho Y. Effects of granulocyte colony-stimulating factor on the hemostatic system in healthy volunteers. Int J Hematol. 1996;63:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Söhngen D, Wienen S, Siebler M, Boogen C, Scheid C, Schulz A, Kobbe G, Diehl V, Heyll A. Analysis of rhG-CSF-effects on platelets by in vitro bleeding test and transcranial Doppler ultrasound examination. Bone Marrow Transplant. 1998;22:1087-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Peter FW, Schuschke DA, Barker JH, Fleischer-Peter B, Hussmann J, Steinau HU. Leukocyte behavior in a free-flap model following chemotherapy and application of granulocyte colony-stimulating factor (GCSF). Microsurgery. 1998;18:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Angelescu M, Hofmann W, Zapletal C, Bredt M, Kraus T, Herfarth C, Klar E. Histomorphological analysis of preservation injury as determinant of graft quality in clinical liver transplantation. Transplant Proc. 1999;31:1074-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Manner M, Shult W, Senninger N, Machens G, Otto G. Evaluation of preservation damage after porcine liver transplantation by assessment of hepatic microcirculation. Transplantation. 1990;50:940-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Post S, Rentsch M, Palma P, Gonzalez AP, Menger MD. Assessment of microhemodynamics after liver transplantation by in vivo microscopy in the rat. Transplant Proc. 1993;25:2597-2598. [PubMed] |
| 18. | Krishna M, Keaveny AP, Genco PV, Rosser BG, Dickson RC, Nguyen JH, Steers JL, Nakhleh RE. Clinicopathological review of 18 cases of liver allografts lost due to bile duct necrosis. Transplant Proc. 2005;37:2221-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003;9:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 265] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. [PubMed] |
| 21. | Howden B, Jablonski P, Grossman H, Marshall VC. The importance of the hepatic artery in rat liver transplantation. Transplantation. 1989;47:428-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Reck T, Steinbauer F, Steinbauer M, Schwille PO, Wittekind C, Hohenberger W, Köckerling F. Impact of arterialization on hepatic oxygen supply, tissue energy phosphates, and outcome after liver transplantation in the rat. Transplantation. 1996;62:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Watanabe T, Suzuya H, Onishi T, Kanai S, Kaneko M, Watanabe H, Nakagawa R, Kawano Y, Takaue Y, Kuroda Y. Effect of granulocyte colony-stimulating factor on bone metabolism during peripheral blood stem cell mobilization. Int J Hematol. 2003;77:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Khettry U, Backer A, Ayata G, Lewis WD, Jenkins RL, Gordon FD. Centrilobular histopathologic changes in liver transplant biopsies. Hum Pathol. 2002;33:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Beaussier M, Wendum D, Fouassier L, Rey C, Barbu V, Lasnier E, Lienhart A, Scoazec JY, Rosmorduc O, Housset C. Adaptative bile duct proliferative response in experimental bile duct ischemia. J Hepatol. 2005;42:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Lichtman SN, Wang J, Clark RL. A microcholangiographic study of liver disease models in rats. Acad Radiol. 1995;2:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Foster PF, Mital D, Sankary HN, McChesney LP, Marcon J, Koukoulis G, Kociss K, Leurgans S, Whiting JF, Williams JW. The use of granulocyte colony-stimulating factor after liver transplantation. Transplantation. 1995;59:1557-1563. [PubMed] |
| 28. | Suzuki A, Takahashi T, Okuno Y, Seko S, Fukuda Y, Nakamura K, Fukumoto M, Konaka Y, Imura H. Liver damage in patients with colony-stimulating factor-producing tumors. Am J Med. 1993;94:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Theocharis SE, Margeli AP, Goutas ND, Horti MG, Karkantaris CS, Kittas CN. Granulocyte colony-stimulating factor administration reverses cadmium-associated inhibition of hepatocyte regeneration. Eur J Gastroenterol Hepatol. 1996;8:805-809. [PubMed] |
| 30. | Theocharis SE, Papadimitriou LJ, Retsou ZP, Margeli AP, Ninos SS, Papadimitriou JD. Granulocyte-colony stimulating factor administration ameliorates liver regeneration in animal model of fulminant hepatic failure and encephalopathy. Dig Dis Sci. 2003;48:1797-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Canales MA, Arrieta R, Gomez-Rioja R, Diez J, Jimenez-Yuste V, Hernandez-Navarro F. Induction of a hypercoagulability state and endothelial cell activation by granulocyte colony-stimulating factor in peripheral blood stem cell donors. J Hematother Stem Cell Res. 2002;11:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |