Published online Aug 7, 2006. doi: 10.3748/wjg.v12.i29.4741
Revised: March 28, 2006
Accepted: April 21, 2006
Published online: August 7, 2006
AIM: To observe the therapeutic effects of liposome-encapsulated adriamycin (LADM) on hepatoma in comparison with adriamycin solution (FADM) and adriamycin plus blank liposome (ADM + BL) administered into the hepatic artery of rats.
METHODS: LADM was prepared by pH gradient-driven method. Normal saline, FADM (2 mg/kg), ADM+BL (2 mg/kg), and LADM (2 mg/kg) were injected via the hepatic artery in rats bearing liver W256 carcinosarcoma, which were divided into four groups randomly. The therapeutic effects were evaluated in terms of survival time, tumor enlargement ratio, and tumor necrosis degree. The difference was determined with ANOVA and Dunnett test and log rank test.
RESULTS: Compared to FADM or ADM + BL, LADM produced a more significant tumor inhibition (tumor volume ratio: 1.243 ± 0.523 vs 1.883 ± 0.708, 1.847 ± 0.661, P < 0.01), and more extensive tumor necrosis. The increased life span was prolonged significantly in rats receiving LADM compared with FADM or ADM+BL (231.48 vs 74.66, 94.70) (P < 0.05).
CONCLUSION: The anticancer efficacies of adriamycin on hepatoma can be strongly improved by liposomal encapsulation through hepatic arterial administration.
- Citation: Sun DS, Chen JH, Ling R, Yao Q, Wang L, Ma Z, Li Y. Treatment of hepatoma with liposome-encapsulated adriamycin administered into hepatic artery of rats. World J Gastroenterol 2006; 12(29): 4741-4744
- URL: https://www.wjgnet.com/1007-9327/full/v12/i29/4741.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i29.4741
Adriamycin (ADM) is extensively used to treat patients with hepatocellular carcinoma (HCC). In vitro studies have verified that the cytocidal effect of ADM depends on the concentration and duration of exposure[1]. Unfortunately, intravenous administration (iv) of a high-dose ADM is often associated with acute toxicity, such as myelosuppression, immunosuppression, and dose-cumulative cardiotoxicity. As a result, drug concentrations in hepatomas are limited. During the past few years, many researchers have reported on drug delivery systems for cancer chemotherapy that aim at the specific targeting of antitumor agents to tumor cells or tumor tissues, thus enhancing the efficacy of chemotherapy as well as reducing its toxicity[2]. Among them, liposomes have drawn much attention for their excellent bioavailability, biodegradability, and targeting, characteristically, to the reticuloendothelial system (RES), especially the liver and spleen[3].
On the other hand, liver primary tumors receive approximately 90% of their blood supply from the hepatic artery. Therefore, transhepatic arterial chemotherapy (TAC) is now widely recognized as an effective means for the treatment of HCC. With TAC, a much higher drug concentration in the tumor-residing zone can be achieved. It was reported that administration of ADM via the hepatic artery (ia) was able to increase the tumor ADM concentration three-fold compared to intravenous (iv) administration[4]. In patients with cancers, the ia administration of ADM reduced the plasma AUC by about 30%[5]. On the basis of these evidences, we hypothesized that a further significant therapeutic effect could be expected by combining TAC and liposome techniques. The current study was carried out to observe the therapeutic effects of liposome-encapsulated adriamycin (LADM) on hepatoma in comparison with the treatment results of adriamycin solution (FADM) and adriamycin plus blank liposome (ADM + BL) administered into the hepatic artery in rats.
Large unilamellar vesicles (LUVs) were prepared by the extrusion method described by Hope et al[6]. Appropriate amounts of lipid mixtures (EPC/Chol, 55:45 mol/mol) in chloroform were dried under a stream of nitrogen gas to form a homogeneous lipid film. The trace amount of solvent was then removed under vacuum overnight. The lipid film was hydrated in a low pH citrate buffer (pH 4.0, 300 mmol/L) by vortex mixing. The resulting multilamellar vesicles (MLVs) were frozen/thawed (liquid nitrogen/55°C) 5 times and extruded 10 times at 55°C through two stacked 100 nm polycarbonate filters (Nuclepore) employing an extrusion device (Lipex Biomembranes, Inc., Vancouver, BC, Canada). ADM was then encapsulated by pH gradient-driven method as described previously[7].
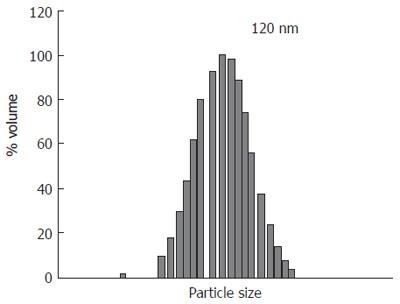
The final product looked like a reddish, semitransparent, colloidal solution. Under electron microscope, LADM showed a global, regular contour with homogeneous size and distribution. The average 120 nm diameters of the liposomes were determined by quasi-elastic light scattering (QELS) using a Nicmp 370 submicrom particle sizer (Sante Burbara, CA, USA) as shown in Figure 1. EPC and Chol were quantified by high performance liquid chromatography (HPLC) method (Table 1). HPLC analysis was performed using a Perkin Elemer HPLC with a PE 200 LC pump, a PE ISS 200 Advanced LC sample Processor, PE 900 Series Interface and an Evaporative Light Scattering Detector (ELSD, Shimadzu). A YMC, PVA 5 m normal phase column was used in this study. The chromatograph was run in a gradient mode at a flow rate of 1 mL/min with the following program, where solution A contained chloroform-isopropanol (50:50, v/v) and solution B contained chloroform-isopropanol-water (36:55:9, v/v).
| Time (min) | 0-3 | 3-11 | 11-11.5 | 11.5-30 |
| A% | 100 | 0 | 0 | 100 |
| B% | 0 | 100 | 100 | 0 |
The drug-to-lipid ratio was 0.25. The drug-embedding ratio was more than 98%. Encapsulation efficiency was calculated as the percentage of ADM incorporated into liposomes relative to the initial amount of ADM in solution as shown in the equation below. The free ADM was separated by elution over a Sephadex G-50 column. Then the free ADM and liposomal ADM were determined by HPLC.
Encapsulation efficiency(%)=[(adriamycin/lipid)Encapsulated/( adriamycin/lipid)total] × 100
The liposomal particle sizes were identical before and after drug loading, and ADM encapsulated liposomes were very stable at room temperature for three days. There was no significant leakage of ADM from liposomes. Just before use, normal saline was added to adjust the final concentration of total ADM to 1.0 mg/mL. Free ADM (FADM) was prepared by dissolution of ADM in normal saline at the same concentration.
Sixty male Wistar rats weighing 230-270 g (mean, 250 g) were provided by Laboratory Animal Center of Fourth Military Medical University and randomly divided into 4 groups, 15 in each. Sumianxin (Changchun Agricultural Pastoral University) was used as anesthetic.
One milliliter of suspension containing 107 Walker-256 (W256) carcinosarcoma cells (Shanghai Medical Industrial Institution) was injected into the thigh muscle of a carrier rat (not included in experimental rats). One week after inoculation, a palpable tumor was found at the injection site. Viable tumor tissue was excised under sterile conditions and soaked in 20 mL of Hanks balanced salt solution. Tissue was cut into approximately 1 mm × 1 mm× 1 mm fragments. Experimental rats were anesthetized with intramuscular injection of Sumianxin at 0.2 mL/kg. Median incision beneath the metasternum was made and the liver was exposed. The tumor fragment was implanted into the left liver lobe.
On the 7th d after tumor implantation, all animals received laparotomy again. The longest (a) and shortest (b) diameters of the tumor were measured. The tumor volume was calculated as
(a×b2)/2
By cannulation method described previously[8], normal saline (NS), FADM, LADM, or ADM mixed with blank liposomes (ADM+BL) were injected into the hepatic artery of rats in groups 1-4 respectively. The dose of ADM in each formulation was 2.0 mg/kg body weight. The concentration of ADM was 1.0 mg/mL.
Tumor growth inhibition: Seven days later, all rats received the third laparotomy. The longest (a) and shortest (b) diameters of the tumor were measured again and the tumor volume after drug administration was calculated. The tumor volume ratio (TVR) was calculated as
TVR = Tumor volume after administration/ Tumor volume before administration
Tumor necrosis degree: Seven random rats in each group were killed and anatomized. Hepatoma was removed completely and fixed in 10% formalin. Three 5 μm thick sections from each tumor were sliced on the maximal transverse plane and mounted on glass slides overnight at room temperature. After HE staining, the sections were examined under microscope. According to the percentage of necrosis area, tumors were graded according to the following criteria: I, 0%-30%; II, 30%-70%; III, 70%-100%.
Increased life span: Survival time of the remaining 8 rats in each group was recorded. The mean survival time of NS group was reckoned as control. Increased life span (% ILS) was calculated as
%ILS = (Mean survival of treated group/ Mean survival of control group - 1)× 100%
SPSS 10.0 for windows was used for statistical analysis. Statistical significance was tested by ANOVA and Dunnett test for tumor growth inhibition, log rank test for survival time. P < 0.05 was considered statistically significant.
No difference in tumor volume was found among the four groups before treatment (P > 0.05, Table 2). After treatment, the tumor grew rapidly in rats which received NS. The mean tumor volume was 31.55 times greater than that before treatment. Metastases were observed in about half of these rats. In contrast, the rate of tumor growth lowered apparently in rats which received FADM or ADM + BL (P < 0.01). No difference between FADM and ADM + BL was observed (P > 0.05). The slowest tumor growth was found in rats which received ia administration of LADM. The mean tumor volume ratio on the 7th day after LADM infusion was 1.243. Statistics indicated that LADM produced a further significant tumor inhibition, as compared with FADM or ADM + BL (P < 0.01). In addition, no metastasis was found in rats receiving LADM.
Under microscope, W256 tumor cells of rats that received NS showed extensive proliferation and active mitoses. Small areas of necroses were observed in the center of tumor tissue accompanied with a few inflammatory cells (Table 3). Moderate to severe necroses were found in tumors of rats that received FADM or ADM+BL. Grade III of tumor necroses was found in 4 of 7 tumors after administration of LADM, including 2 cases of complete tumor necrosis.
Compared with the survival time (11.88 d) of control rats, survival time was greatly prolonged in rats that received LADM (mean, 39.38 d), or FADM (mean, 20.75 d), or ADM+BL (mean, 23.13 d) (Table 4). LADM prolonged the life span by 231.48%, which was significantly longer than FADM (74.66%) and ADM + BL (94.70%)(P < 0.01).
Hepatocellular carcinoma (HCC) is by far the third most common cause of cancer mortality throughout the world, especially in the Asian-Pacific region, and its incidence continues to increase. On the other hand, HCC has a high expression rate of multi-drug resistance gene (MDR1) and consequently high levels of P-glycoprotein (P-gp), resulting in its insensitiveness to most chemotherapeutic drugs[9]. Few agents show response rates (RRs) above 20%. ADM is one of the most successful anticancer agents to date for the treatment of HCC. Early phase II trials and case series reported RRs ranging from 25% to 100% with single-agent ADM[10]. However, despite its excellent antitumour activity, ADM has a relatively low therapeutic index, and its clinical utility is frequently limited due to acute and chronic toxicities such as myelosuppression, immunosuppression, and dose-cumulative cardiotoxicity. A retrospective analysis of three phase III trials of patients treated with ADM in combination with other cytotoxic agents or radiation therapy demonstrates that ADM-associated cardiac events may occur more frequently and at lower cumulative doses than previously reported[11]. Encapsulation of ADM into liposomes may minimize the side effects and enhance the antitumor efficacy by altering its pharmacokinetics and biodistribution pattern. Growing evidence supports the use of LADM as a substitute for ADM to increase the therapeutic index by maintaining the antitumor efficacy while improving the safety profile[12,13]. After intravenous (iv) administration, LADM is predominantly uptaken by the reticuloendothelial system (RES), especially by the liver and spleen[14].
The results of our experiments demonstrated that the antitumor activity of ADM could be markedly enhanced when the drug was encapsulated in liposomes and administered into the hepatic artery. Equivalent or similar effects could not be obtained using FADM or ADM + BL. Compared with FADM or ADM + BL, LADM produced a more significant tumor inhibition and more extensive tumor necrosis. The average tumor volume on the 7th day after treatment with LADM was 0.105 cm3, about 70% of 0.149 cm3 of the volume after treatment with FADM. The survival time of rats that received LADM was evidently increased. Compared with saline controls, LADM prolonged the life span by 231.48%, which was significantly longer than FADM (74.66%) and ADM + BL (94.70%).
The likely explanations for the improved therapeutic activity of LADM are as follows. First, W256 carcinosarcoma proliferated rapidly with abundant vasculo-genesis, which strongly increased the uptake of LADM by tumor tissues after ia administration. In addition, the deformity of tumor vessels caused significant retention of LADM in tumor tissues. Secondly, it has been testified that the cytocidal effect of ADM depends on the concentration and duration of exposure[1]. Increasing ADM concentration in tumor cells or slowing its elimination could certainly enhance its antitumor efficacy, both of which could be met after ia administration of LADM. Thirdly, encapsulating the drug into liposomes might modify its distribution pattern in tissues. After being administered into rats, liposomes were taken up selectively by RES, such as the liver, spleen, and bone marrow[14]. Accumulation of biodegradable liposomes with associated drugs in Kupffer cells would create a gradient of drug concentration for a massive and prolonged diffusion of the free drug towards neoplastic tissues. Fourthly, the tiny size of the liposome could increase its contact areas significantly, which might result in an apparent improvement in its bioavailability.
We did not include iv administration in the present experiment because the ia route is much more efficient than iv in the treatment of liver malignancies. Different from the liver parenchyma, which receives more than 70% of its blood supply from the portal vein and the rest from the hepatic artery, hepatoma receives approximately 90% of its blood supply from the hepatic artery. The uniqueness of liver blood supply determines the major difference between the two routes of administration. The difference does not warrant repeat comparison in our study.
Our experiments support the hypothesis that the therapeutic effect of TAC could be dramatically enhanced by combination with nanotechnology. We conclude that liposomes can be used as a promising drug carrier in TAC for the treatment of liver primary and metastatic tumors.
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
| 1. | Rupniak HT, Whelan RD, Hill BT. Concentration and time-dependent inter-relationships for antitumour drug cytotoxicities against tumour cells in vitro. Int J Cancer. 1983;32:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Kim CK, Lim SJ. Recent progress in drug delivery systems for anticancer agents. Arch Pharm Res. 2002;25:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 258] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Ridge JA, Collin C, Bading JR, Hancock C, Conti PS, Daly JM, Raaf JH. Increased adriamycin levels in hepatic implants of rabbit Vx-2 carcinoma from regional infusion. Cancer Res. 1988;48:4584-4587. [PubMed] |
| 5. | Eksborg S, Cedermark BJ, Strandler HS. Intrahepatic and intravenous administration of adriamycin--a comparative pharmacokinetic study in patients with malignant liver tumours. Med Oncol Tumor Pharmacother. 1985;2:47-54. [PubMed] |
| 6. | Hope MJ, Nayar R, Mayer LD, Cullis PR. Reduction of liposome size and preparation of unilamellar vesicles by extrusion techniques. In: Liposome Technology. Vol I. 1st ed. Gregoriadis ed 1993; 123-131. |
| 7. | Cullis PR, Hope MJ, Bally MB, Madden TD, Mayer LD, Fenske DB. Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochim Biophys Acta. 1997;1331:187-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 159] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Zou YY, Ueno M, Yamagishi M, Horikoshi I, Yamashita I, Tazawa K, Gu XQ. Targeting behavior of hepatic artery injected temperature sensitive liposomal adriamycin on tumor-bearing rats. Sel Cancer Ther. 1990;6:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Huang CC, Wu MC, Xu GW, Li DZ, Cheng H, Tu ZX, Jiang HQ, Gu JR. Overexpression of the MDR1 gene and P-glycoprotein in human hepatocellular carcinoma. J Natl Cancer Inst. 1992;84:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer. 2004;40:1474-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869-2879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1494] [Cited by in RCA: 1642] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 12. | Schmidinger M, Wenzel C, Locker GJ, Muehlbacher F, Steininger R, Gnant M, Crevenna R, Budinsky AC. Pilot study with pegylated liposomal doxorubicin for advanced or unresectable hepatocellular carcinoma. Br J Cancer. 2001;85:1850-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Safra T. Cardiac safety of liposomal anthracyclines. Oncologist. 2003;8 Suppl 2:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Bao A, Goins B, Klipper R, Negrete G, Phillips WT. Direct 99mTc labeling of pegylated liposomal doxorubicin (Doxil) for pharmacokinetic and non-invasive imaging studies. J Pharmacol Exp Ther. 2004;308:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |