Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.3994
Revised: October 5, 2005
Accepted: October 9, 2005
Published online: July 7, 2006
AIM: To study the direct correlation between gastric dysrhythmias and in vivo gastric muscle tone.
METHODS: Five healthy dogs were implanted with 4 pairs of electrodes along the greater curvature, with a strain gauge (SG) being sutured parallel to the distal electrodes (2 cm above the pylorus). Intravenous vasopressin was given to induce gastric dysrhythmia. The percentage of regular slow waves and SG energy were calculated.
RESULTS: (1) the regularity of gastric myoelectric activity (GMA) was reduced during and after infusion of vasopressin; (2) SG energy was significantly decreased during the infusion of vasopressin; (3) the decrease in SG energy was well correlated with the reduction in GMA regularity; (4) SG energy was negatively correlated with bradygastria and tachygastria.
CONCLUSION: Vasopressin inhibits gastric contractions and impairs gastric slow waves; gastric dysrhythmias are associated with the reduced antral muscle contractions, and are indicative of antral hypomotility.
-
Citation: Xing J, Qian L, Chen J. Experimental gastric dysrhythmias and its correlation with
in vivo gastric muscle contractions. World J Gastroenterol 2006; 12(25): 3994-3998 - URL: https://www.wjgnet.com/1007-9327/full/v12/i25/3994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.3994
Gastric motility is under the control of gastric myoelectric activity (GMA). Normally GMA originates from the junctional area of the fundus and proximal stomach, propagates aborally, controlling the frequency and direction of gastric contractions. Disturbances in GMA, so-called gastric dysrhythmias, have been frequently observed in patients with various diseases including gastroparesis[1], dyspepsia[2], anorexia nervosa[3], gastro esophageal reflux diseases (GERD)[4], motion sickness[5,6] and pregnancy[7], etc. Gastric dysrhythmias have also been linked to gastrointestinal symptoms such as nausea and vomiting[5,6], and improvement of gastric dysrhythmias seems to be associated with relief of such symptoms[5,8].
Gastric dysrhythmias are classified into tachygastria (frequency higher than normal), bradygastria (frequency lower than normal) and arrhythmia (no rhythmic activity), based on the dominant frequency of GMA. Although it is believed that gastric motility could be affected inevitably, the relationship between gastric dysrhythmias and gastric motility has not well been established. Various techniques including electrogastrography (EGG), gastric emptying and simultaneous recording of EGG and intraluminal pressure have been applied, and available evidence suggests that tachygastria may be associated with hypo-gastric motility[9-12], while the effect of bradygastria on gastric motility remains controversial[13,14]. The association between specific gastric dysrhythmia and gastric muscle contractions has never been carefully investigated.
To address these issues, in this study we specifically evaluated: (1) the effect of intravenous vasopressin on GMA; (2) the correlation between gastric muscle tone and GMA; (3) the effect of experimentally-induced gastric dysrhythmias on gastric muscle contractions, in a canine model.
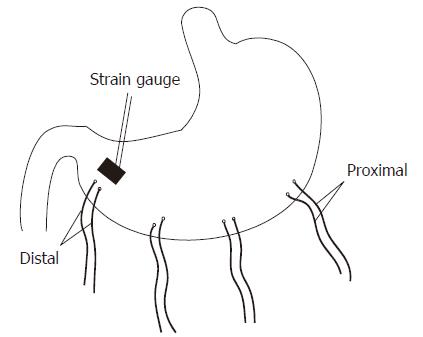
Seven healthy female hound dogs (15-22 kg) were anesthetized with intravenous infusion of thiopental sodium (20 mg/kg) and maintained with inhalation of isoflurane (1%-2%). A midline laparotomy was performed, and four pairs of temporary cardiac pacing wires (A&E Medical, Farmingdale, NJ) were implanted under the serosal surface along the greater curvature of the stomach. The most distal pair was 2 cm above the pylorus, and the distance between adjacent pairs of electrodes was 4 cm (Figure 1). The electrodes in each pair were 1 cm apart. The electrodes were affixed to the gastric serosa by un-absorbable suture in the seromuscular layer of the stomach. The wires were brought out through the anterior abdominal wall, channeled subcutaneously along the right side of the trunk, and placed outside the skin for the attachment for recording gastric myoelectric activity. A strain gauge was sutured on the serosal wall parallel to the most distal pair of electrodes (Figure 1).
The study was initiated about 10 d after the surgery. The protocol was approved by the Animal Use and Care Committee of the University of Texas Medical Branch at Galveston, Texas, USA.
Experiments were performed in overnight fasted, fully recovered animals. One study session was performed in each animal. Vasopressin was administered intravenously to induce gastric dysrhythmias in each session. In brief, after a 20 min baseline recording, Vasopressin (0.5 U/kg) was infused continuously for 20 min, followed by another two 20 min recovery periods. GMA and strain gauge signal were recorded simultaneously throughout the session.
Gastric electrical activity and gastric muscle tone were recorded using a multi-channel recorder system (Acknowledge, Biopac System, Santa Barbara, CA). All signals were displayed on a computer monitor and saved on a hard disk by an HP Pentium IV PC. For GMA, the low and high cutoff frequencies of the amplifier were 0.05 Hz and 35 Hz, and the sampling frequency was 20 Hz. The recordings were then low-pass filtered with a frequency of 1 Hz and re-sampled at a frequency of 24 Hz to reduce the volume of data and potential artifacts before the final review.
Regularity of GMA: The percentage of 4- to 6-cpm slow waves is a quantitative assessment of the regularity of the GMA measured from the EGG. It was defined as the percentage of time during which normal 4- to 6-cpm gastric slow waves were observed in a specific EGG recording. The percentage of normal 4- to 6-cpm slow waves was computed from the running power spectra of the GMA using an adaptive spectral analysis method[15]. One power spectrum was generated for every 2 min of EGG data, and the spectral peaks in each spectrum were examined visually. A spectrum was defined as normal if it had a clear peak in the 4- to 6-cpm range. The percentage of regular 4- to 6-cpm slow waves was determined by computing the ratio between the numbers of normal and total spectra.
Gastric dysrhythmias: The percentage of time of gastric dysrhythmias was also computed using running spectral analysis. Bradygastria was defined as a frequency of 0.5-4.0 cpm. Tachygastria was defined as a frequency of 6.0-15.0 cpm. Arrhythmia was defined as any irregular rhythm.
Gastric muscle tone: Reflected by the energy of the strain gauge signals, which were derived by calculating the area under the curve.
GMA regularity within each session was compared with One-Way ANOVA. The correlation between gastric muscle tone and gastric dysrhythmias was analyzed with Pearson’s correlation test. All data were presented as mean ± SE. P < 0.05 was taken as significance.
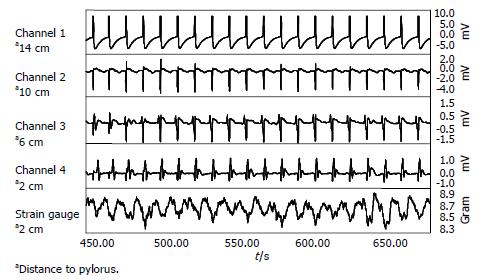
Intrinsic, distally propagating gastric slow waves were observed in all animals at a mean frequency of 5.6 ± 0.3 cpm, with a range of 4.8-6.2 cpm. Rhythmic variations in gastric muscle tone were consistently present, and coupled with each gastric slow waves at an identical frequency (Figure 2).
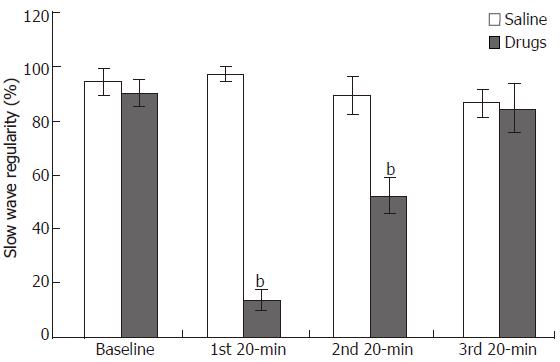
The regularity of GMA was 90.1%± 5.0% at baseline. It was reduced to 13.9% ± 3.9% and 52.2%± 6.4 % during the following two 20-min periods with and after infusion of vasopressin (P < 0.01), and returned to 84.7% ± 9.2% during the last 20-min period (Figure 3).
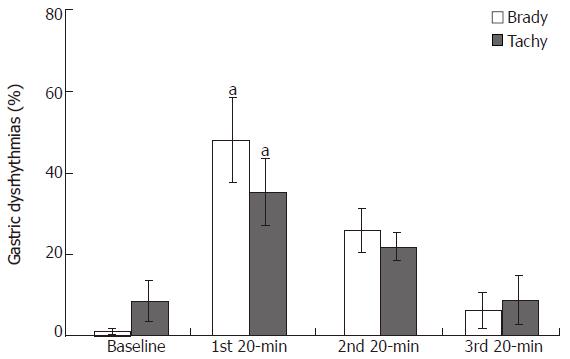
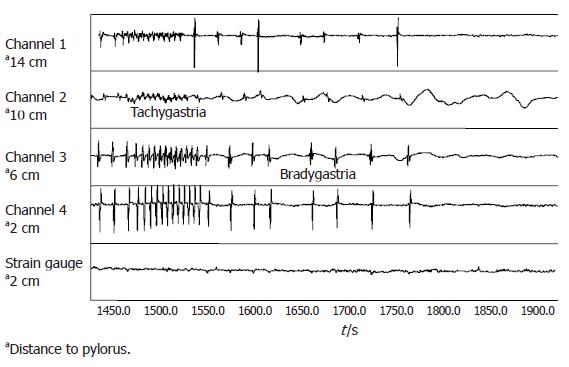
The decrease in the regularity of GMA was attributed to an increase in both bradygastria (48.0% ± 10.4% vs 1.1% ± 0.8 % and tachygastria (35.4% ± 8.2 % vs 8.7% ± 5.0%) (Figure 4).
The strain gauge energy was significantly decreased during the 20-min infusion of vasopressin. The total energy was 48.4 ± 1.3 dB at baseline, and decreased to 36.6 ± 4.5 dB, 44.5 ± 4.3 dB, 45.8 ± 1.9 dB during the following consecutive three 20-min periods during and after the infusion (vs baseline, P < 0.05).
The decrease in strain gauge energy was positively correlated with the decrease of GMA regularity (r = 0.96, P < 0.05). Gastric muscle contractions was reduced or disappeared during periods of bradygastria or tachygastria (Figure 5). Strain gauge energy was negatively correlated with bradygastria (r = -0.96, P < 0.05) and tachygastria (r = -0.95, P > 0.05).
Through this study we have found: (1) Intravenous vasopressin at proper doses consistently produced gastric dysrhythmias; (2) strain gauge implanted on gastric wall could reliably detect gastric muscle contractions; gastric muscle contractions were coupled with intrinsic gastric slow waves, and each gastric slow wave was capable of inducing a change in gastric muscle tone; (3) gastric muscle contractions was positively correlated with the regularity of the gastric slow waves; and (4) the reduction or disappearance in gastric muscle contractions was associated with both bradygastria and tachygastria.
Considering the fact that all muscle contractions are coupled and superimposed with myoelectric activities, it is strongly believed that gastric dysrhythmias could cause disturbances in gastric motor functions[16]. A larger number of clinical studies have been performed recently, and indeed, gastric dysrhythmias have been observed in various motility disorders like gastroparesis, functional dyspepsia and motion sickness, etc[1-7]. In patients with gastroparesis, gastric emptying scintigraphy and EGG were performed concurrently, and it was found that postprandial gastric dysrhythmias correlated with delays in solid phase gastric emptying[17]. Gastric dysrhythmias may also be in association with the occurrence of GI symptoms[5,6]. We have noted on many occasions that degeneration of GMA preceded the occurrence of nausea and vomiting in our previous canine studies. Other investigators also reported similar findings[5,6,18], and all these indicate a possible causative role for the dysrhythmia in the production of nausea and vomiting.
Only few studies have been conducted to qualitatively assess the association between gastric dysrhythmias and gastric motility and there have been no quantitative studies. The first report on tachygastria in humans was published by Telander et al[11]. They reported a 5 month-old male infant suffering from severe gastric retention and symptoms of intractable nausea, vomiting and weight loss. The symptoms were attributed to impaired motor function of the stomach. The patient underwent resection of the distal ¾ of the stomach. The excised tissues were studied in vitro by means of intracellular electrodes, and abnormally fast slow waves (> 5 cpm) were detected[11]. The association between gastric dysrhythmia and gastric motor disorders was further substantiated by other studies. You et al[12] observed tachygastria in a 26-year-old woman with persistent nausea, vomiting and abdominal pain who was found to have severe impairment of antral motor functions. In contrast to tachygastria, data on bradygastria are inconsistent. Van Der Schee et al observed bradygastria in dogs and found that it was correlated with strong antral contractions[13], while Abell et al[14] induced bradygastria in humans with glucagon, and found that it was associated with absence of antral contraction.
In our present study, we attached a stain gauge to the site of recording electrodes, which allows us to study the direct correlation of gastric contractions and GMA. In comparison to the traditional intraluminal manometry, strain gauge is more sensitive and more direct in the detection of subtle contractions of gastric wall than any other methods. In addition, gastric dysrhythmias in this study were assessed quantitatively rather than qualitatively in the previous studies. Our results indicate that both tachygastria and bradygastria are negatively associated with gastric muscle contractile activity, which is consistent with most of the previous report[11-14].
The exact mechanisms causing gastric dysrhythmias have not been fully understood, though it is well known that GMA is generated by interstitial cells of Cajal, and is influenced by central and autonomic nerve systems, certain hormones and peptides. Tachygastria and bradygastria are different. Tachygastria usually originates from distal stomach, and can be considered as an ectopic rhythm, while transient or persistent bradygastria usually originates from the region of the normal pacemaker at a reduced frequency. Gastric dysrhythmia can be induced experimentally with various agents including vasopressin[14,19-21]. Vasopressin is a peptide released into the peripheral circulation from the pituitary during experimental motion sickness, and has been confirmed to induce gastric and intestinal dysrhythmia and symptoms of nausea and vomiting[20-22]. An elevated plasma level of vasopressin was reported in postsurgical patients with impaired gastrointestinal motility[23]. Vasopressin was known to reduce mesenteric blood flow, which was frequently used in patients with esophageal variceal bleeding[24], and this vasoconstrictive effect might be the mechanism involved in the induction of GI dysrhythmias.
Gastric myoelectric activity can be recorded in several ways including intraluminal electrodes, surface electrogastrogram and serosal electrodes. Placement of intraluminal electrodes is invasive and uncomfortable. It is very difficult to direct the electrodes to specific sites of stomach and electrodes can be easily dislodged. EGG is a convenient summation technique with various disadvantages: (1) derived data on frequency and power are not strictly region specific; (2) susceptible to a variety of artifacts; (3) cannot discriminate chaotic rhythm from uncoupling; and (4) susceptible to myoelectric interference from other gut organs, and cutaneously acquired signals may not be gastric in origin[25]. Serosal recordings, on the other hand, do not have these potential technical problems, and is the most reliable in vivo GMA recording except for the need for surgery. This method has allowed us to put the recording electrodes and strain gauge at the same location, thus providing an excellent tool for the study of the direct correlation of dysrhythmias and gastric contraction, which has never been done before.
Another interesting finding from this study is that under control conditions each gastric slow wave seems capable of inducing a certain level of mechanical contractions in gastric muscle. These contractions are so subtle that they could only be reflected by strain gauge, other than other techniques, including intraluminal manometry. This observation is inconsistent with traditional view that only gastric slow waves superimposed with spike activities are capable of inducing gastric contractions.
In summary, in this study we have observed that intravenous administration of vasopressin could impair gastric slow waves and gastric muscle contractions. Experimentally induced tachygastria and bradygastria are negatively correlated with the gastric muscle contractions, suggesting that gastric dysrhythmias, either tachygastria or bradygastria, may be indicative of gastric hypomotility.
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L
| 1. | Chen J, McCallum RW. Gastric slow wave abnormalities in patients with gastroparesis. Am J Gastroenterol. 1992;87:477-482. [PubMed] |
| 2. | Chou LT, Wu CY, Chen HP, Chang CS, Wong PG, Ko CW, Chen GH. The correlation of depression and gastric dysrhythmia in functional dyspepsia. J Clin Gastroenterol. 2001;33:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Abell TL, Malagelada JR, Lucas AR, Brown ML, Camilleri M, Go VL, Azpiroz F, Callaway CW, Kao PC, Zinsmeister AR. Gastric electromechanical and neurohormonal function in anorexia nervosa. Gastroenterology. 1987;93:958-965. [PubMed] |
| 4. | Cucchiara S, Salvia G, Borrelli O, Ciccimarra E, Az-Zeqeh N, Rapagiolo S, Minella R, Campanozzi A, Riezzo G. Gastric electrical dysrhythmias and delayed gastric emptying in gastroesophageal reflux disease. Am J Gastroenterol. 1997;92:1103-1108. [PubMed] |
| 5. | Hasler WL, Kim MS, Chey WD, Stevenson V, Stein B, Owyang C. Central cholinergic and alpha-adrenergic mediation of gastric slow wave dysrhythmias evoked during motion sickness. Am J Physiol. 1995;268:G539-G547. [PubMed] |
| 6. | Stern RM, Koch KL, Stewart WR, Lindblad IM. Spectral analysis of tachygastria recorded during motion sickness. Gastroenterology. 1987;92:92-97. [PubMed] |
| 7. | Koch KL, Stern RM, Vasey M, Botti JJ, Creasy GW, Dwyer A. Gastric dysrhythmias and nausea of pregnancy. Dig Dis Sci. 1990;35:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 124] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Uijtdehaage SH, Stern RM, Koch KL. Effects of scopolamine on autonomic profiles underlying motion sickness susceptibility. Aviat Space Environ Med. 1993;64:1-8. [PubMed] |
| 9. | Chen JD, Pan J, McCallum RW. Clinical significance of gastric myoelectrical dysrhythmias. Dig Dis. 1995;13:275-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Abell TL, Camilleri M, Hench V, Malagelada JR. Gastric electromecahnical function and gastric emptying in diabetic gastroparesis. Eur J Gastroenterol Hepatol. 1991;3:163-167. |
| 11. | Telander RL, Morgan KG, Kreulen DL, Schmalz PF, Kelly KA, Szurszewski JH. Human gastric atony with tachygastria and gastric retention. Gastroenterology. 1978;75:497-501. [PubMed] |
| 12. | You CH, Chey WY, Lee KY, Menguy R, Bortoff A. Gastric and small intestinal myoelectric dysrhythmia associated with chronic intractable nausea and vomiting. Ann Intern Med. 1981;95:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | van der Schee EJ, Grashuis JL. Contraction-related, low-frequency components in canine electrogastrographic signals. Am J Physiol. 1983;245:G470-G475. [PubMed] |
| 14. | Abell TL, Malagelada JR. Glucagon-evoked gastric dysrhythmias in humans shown by an improved electrogastrographic technique. Gastroenterology. 1985;88:1932-1940. [PubMed] |
| 15. | Chen J. A computerized data analysis system for electrogastrogram. Comput Biol Med. 1992;22:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Parkman HP, Hasler WL, Barnett JL, Eaker EY. Electrogastrography: a document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol Motil. 2003;15:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 208] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Stern RM, Koch KL, Leibowitz HW, Lindblad IM, Shupert CL, Stewart WR. Tachygastria and motion sickness. Aviat Space Environ Med. 1985;56:1074-1077. [PubMed] |
| 19. | Caras SD, Soykan I, Beverly V, Lin Z, McCallum RW. The effect of intravenous vasopressin on gastric myoelectrical activity in human subjects. Neurogastroenterol Motil. 1997;9:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Kim MS, Chey WD, Owyang C, Hasler WL. Role of plasma vasopressin as a mediator of nausea and gastric slow wave dysrhythmias in motion sickness. Am J Physiol. 1997;272:G853-G862. [PubMed] |
| 22. | Xu LH, Koch KL, Summy-Long J, Stern RM, Seaton JF, Harrison TS, Demers LM, Bingaman S. Hypothalamic and gastric myoelectrical responses during vection-induced nausea in healthy Chinese subjects. Am J Physiol. 1993;265:E578-E584. [PubMed] |
| 23. | Chen JD, McCallum RW. Clinical applications of electrogastrography. Am J Gastroenterol. 1993;88:1324-1336. [PubMed] |
| 24. | Cochrane JP, Forsling ML, Gow NM, Le Quesne LP. Arginine vasopressin release following surgical operations. Br J Surg. 1981;68:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Amaris MA, Sanmiguel CP, Sadowski DC, Bowes KL, Mintchev MP. Electrical activity from colon overlaps with normal gastric electrical activity in cutaneous recordings. Dig Dis Sci. 2002;47:2480-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |