Published online Jun 7, 2006. doi: 10.3748/wjg.v12.i21.3438
Revised: December 28, 2005
Accepted: February 18, 2006
Published online: June 7, 2006
AIM: To detect the biological characters of the SGC7901 gastric cancer cell-dendritic cell fusion vaccines.
METHODS: The suspending living SGC7901 gastric cancer cells and dendritic cells were induced to be fusioned by polyethylene glycol. Pure fusion cells were obtained by selective culture with the HAT/HT culture systems. The fusion cells were counted at different time points of culture and their growth curves were drawn to reflect their proliferative activities. The fusion cells were also cultured in culture medium to investigate whether they could grow into cell clones. MTT method was used to test the stimulating abilities of the fusion cells on T lymphocytes’ proliferations. Moreover, the fusion cells were planted into nude mice to observe whether they could grow into new planted tumors in this kind of immunodeficiency animals.
RESULTS: The fusion cells had weaker proliferative activity and clone abilities than their parental cells. When they were cultured, the counts of cells did not increase remarkably, nor could they grow into cell clones in culture medium. The fusion cells could not grow into new planted tumors after planted into nude mice. The stimulating abilities of the fusion cells on T lymphocytes’ proliferations were remarkably increased than their parental dendritic cells.
CONCLUSION: The SGC7901 gastric cancer cell-dendritic cell fusion vaccines have much weaker proliferative abilities than their parental cells, but they keep strong abilities to irritate the T lymphocytes and have no abilities to grow into new planted tumors in immunodeficiency animals. These are the biological basis for their anti-tumor biotherapies.
- Citation: Zhang K, Gao PF, Yu PW, Rao Y, Zhou LX. Study on biological characters of SGC7901 gastric cancer cell-dendritic cell fusion vaccines. World J Gastroenterol 2006; 12(21): 3438-3441
- URL: https://www.wjgnet.com/1007-9327/full/v12/i21/3438.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i21.3438
With the development of modern immune research, biotherapy has brought new hopes for gastric cancer patients to conquer this kind of malignant tumors[1]. The anti-tumor vaccines based on dendritic cells (DC) play important roles in anti-tumor biotherapies[2]. Researchers obtain the cancer cells’ antigens by various methods to irritate the dendritic cells, and increase the antigen presenting and immune stimulating abilities of dendritic cells[3,4]. Researchers usually destroy the cancer cells by frozen or ultrasound[5] methods. The components of the destroyed cancer cells were used as cancer antigens to irritate the dendritic cells. Many other researchers transmitted the cancer cell related mRNA into dendritic cells [6] or mix-cultured the cancer cells and dendritic cells[7]. They also attempted to improve the antigen presenting and immune stimulating abilities of dendritic cells by these methods. Although all these methods could partially enhance dendritic cells’ capabilities and anti-tumor immune function of cancer patients, they still have some limitations. In recent years, methods to induce the dendritic cells and cancer cells to be fusioned together have been developed in order to investigate whether the fusion cells have stronger immune stimulating abilities than normal dendritic cells. Gastric cancer cells have no strong and specific tumor antigens, which makes it difficult for gastric cancer biotherapies. If gastric cancer cells and dendritic cells could be fusioned together, the cancer cell antigens might be presented more efficiently and more obvious anti-tumor effects might be achieved. In this research, the gastric cancer cells and dendritic cells were induced to be fusioned together by cell fusion technology. We detected the fusion cells’ biological characters so to provide experimental data for gastric cancer biotherapies.
The reagents used included rhGM-CSF (Promega Co. Ltd, USA), rhIL-4 (R&D System Co. Ltd), TNF-a(R&D System Co. Ltd), Polyethylene glycol (Sigma, USA), Hypoxanthine (Sigma, USA), Aminopterin (Sigma, USA), Thymidine (Sigma, USA).
The frozen stored SGC7901 gastric cancer cells were cultured in the DMEM culture medium, and the suspending living cancer cells were prepared for the fusion process. The peripheral blood mononuclear cells were separated from venous blood of gastric cancer patients by use of lymphocytes separating solution. rhGM-CSF (100 ng/mL), rhIL-4 (50 ng/mL) and TNF-α(20 ng/mL) were added into the cultured peripheral blood mononuclear cells to induce them to transform into dendritic cells.
Suspending living SGC7901 gastric cancer cells and dendritic cells were mix-cultured with the ratio of 10:1. The two kinds of parental cells were induced to be fusioned by polyethylene glycol and selectively cultured by HAT/HT culture solutions. After the culture process, we obtained pure fusion cells as anti-tumor vaccines. Simultaneously, the suspending living SGC7901 gastric cancer cells and dendritic cells were mix-cultured with the same ratios, but were not induced to the fusion process. This group was designed as control group.
The obtained pure fusion cells (cell density 1.2×106/mL) were inoculated into the culture media on the culture slabs. During the culture process, we counted the cell numbers daily. After fourteen days, we drew the growth curves of cells. The SGC7901 cancer cells’ and dendritic cells’ growth curves were drawn at the same time respectively, and these curves were served as control.
The suspending living fusion cells (cell density 1×104/mL) were inoculated into the agar culture medium on twenty-four holes culture slabs. The cell numbers in each hole were 1000, 2000 and 10 000 respectively. After two weeks’ cultivation, the cell clones which included more than 50 cells were counted under the microscopes. At the same time, the clone abilities of SGC7901 gastric cancer cells and dendritic cells were tested by the same methods.
T lymphocytes were separated from peripheral blood of gastric cancer patients by using nylon hair. The cell densities of T lymphocytes, fusion cells and mix-cultured dendritic cells were adjusted to 5 × 106/mL. The fusion cells and mix-cultured dendritic cells were cultured on the culture slabs with 96 holes. T lymphocytes were added. The ratios of T lymphocytes to fusion cells and mix-cultured dendritic cells were 100:1, 50:1, 10:1 and 1:1 respectively. For each ratio, six holes were repeated in order to decrease the systemic errors. After sixty hours’ cultivation, 3H-TdR was added into each hole on the slabs (1Ci per hole). Twelve hours later, the CPM values were tested by Beckman liquid scintillation counting. The culture groups which included T lymphocytes only or DMEM culture medium were designed as control groups.
The suspending living pure fusion cells (cell density 1×109/mL, cell number 5×108) were planted subcutaneously into the back of the BALB/c nude mice. The injections were performed every three days for about two weeks. We observed whether the planted fusion cells could grow into new planted tumors in the nude mice, and the percentages of planted tumor cells growing into new planted tumors were tested. At the same time, the SGC7901 gastric cancer cells were planted into other groups of nude mice. These groups were designed as control groups.
Data were imported into Systat software (SPSS) for statistical computations and graphing. One-way analysis of variance (ANOVA), independent-samples T test and Chi-Square tests were used to evaluate the differences between groups. P < 0.05 was considered as significant.
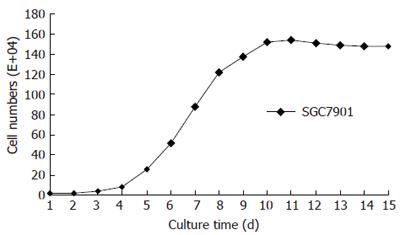
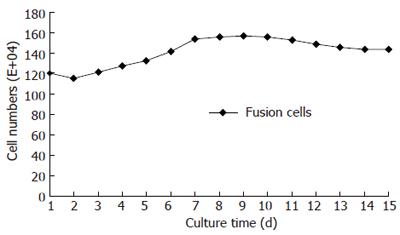
We obtained the fusion cells after the selective culture process. The fusion cells’ phenotypes and morphological characters were examined by flow cytometer and histological methods. The parental SGC7901 gastric cancer cells proliferated fast during their cultivation. After the subculture, the cancer cells stayed in proliferative delitescence for a short period, and then they entered into the logarithmic growth period with cell number increasing quickly. The cancer cells’ proliferation slowed down and their number decreased gradually (Figure 1). Dendritic cells had much weaker proliferative abilities than SGC7901 cancer cells. During their cultivation, the cells’ number did not increase remarkably. Like the parental dendritic cells, fusion cells also had weak proliferative abilities with a flat growth curve. This indicated that fusion cells’ proliferative characters were much similar to parental dendritic cells (Figure 2).
Clone abilities effectively reflect the proliferation of single cells. If a kind of cell could grow into numerous cell clones in the agar culture medium, it indicates these cells have strong abilities to proliferate. In our study, we found that SGC7901 gastric cancer cells had strong proliferative abilities. They grew into numerous cell clones fast. Like the parental dendritic cells, fusion cells also had much weaker proliferations. During their cultivation, they could not grow into visible cell clones.
The abilities of fusion cells to irritate T lymphocytes were much stronger than the mix-cultured DC group (P < 0.01) (Table 1).
After injected subcutaneously into the back of the BALB/c nude mice, the SGC7901 gastric cancer cells grew into new planted tumors quickly, whereas the fusion cells could not grow into new tumors in nude mice, even with a higher cell density and injection frequency, suggesting that fusion cells had no abilities to proliferate and grow into new planted tumors in immunodeficiency animals.
Cell number and growth curves could directly reflect cell proliferative characters[8]. Clone ability is an important criterion to judge cells’ growing capabilities[9]. The stronger the growth powers, the more cell clones could be formed. Cancer cells had strong abilities to proliferate. In our research we found that when the SGC7901 gastric cancer cells were subcultured, they quickly grew into the logarithmic proliferation period and the cell number increased remarkably[10]. After a short period the cell number was doubled. They grew into clearly cell clones only after seven days’ cultivation. On the contrary, dendritic cells had much weaker proliferative abilities with a flat growth curve. There were no remarkable increases of the cell number during their cultivation, nor did cell clones formed. We found that fusion cells’ growth characters were similar to their parental dendritic cells. They also had much weaker proliferative abilities and could not grow into cell clones during their cultivation. The cell number maintained a stable level and the growth curves were just a flat line. The SGC7901-DC fusion cells kept more biological characters of their parental dendritic cells. Different from their parental cancer cells, the fusion cells had no abilities to proliferate fast and grow into cell clones. These indicate that fusion cells were safe to be used as anti-tumor vaccines, which are biological basis for fusion cells research in cancer treatment.
After tumor antigens were presented by the dendritic cells, T lymphocytes were irritated and showed anti-tumor biological effects[11]. T lymphocytes in vivo can be divided into several sub-groups which have different phenotypes and biological effects. The most important sub-groups are CD4+ T lymphocytes (Th) and CD8+ T lymphocytes (Tc/CTL)[12]. The in-activated pre-Tc cells were irritated and became active Tc cells in vivo by the tumor antigens and CD4+ Th cells. These activated Tc cells exerted anti-tumor biological effects by the double signal approaches[13]. The cell number and activities of T lymphocytes directly affected anti-tumor immune responses of cancer patients[9]. Our purposes of modifying dendritic cells as anti-tumor vaccines were to increase their abilities of stimulating T lymphocytes’ proliferation and their activities[14,15]. Some researchers reported that when the tumor antigen related genes were transmitted into dendritic cells T lymphocytes in vivo could be strongly irritated to become CD4+ Th cells and CD8+ Th cells [16,17]. In our research, we found that the SGC7901-DC fusion cells could strongly irritate T lymphocytes’ proliferation, while the mix-cultured dendritic cells could not. This indicated that fusion cells had much stronger abilities to activate anti-tumor immune responses. So, fusion cells are possible to be used as anti-tumor vaccines.
New planted tumors in immunodeficiency animals are perfect models for oncology researches[18]. We injected the SGC7901 cancer cells and SGC7901-DC fusion cells into different groups of nude mice and found that SGC7901 cancer cells could grow into new planted tumors quickly but the fusion cells could not, even when much higher cell densities and more injection frequencies were applied. This implied that SGC7901-DC fusion cells had no abilities to grow into new planted tumor in nude mice. This is the most important difference between the parental SGC7901 cells and the SGC7901-DC fusion cells. This character together with the growth curves and clone abilities indicated that fusion cells were safe when used as anti-tumor vaccines in vivo and in vitro.
The strong abilities of SGC7901-DC fusion cells to irritate T lymphocytes’ proliferation and the characters that the fusion cells could not grow into new planted tumors in vivo are the precondition of anti-tumor biological therapy.
S- Editor Wang J L- Editor Zhu LH E- Editor Zhang Y
| 1. | Tong QS, Zheng LD, Chen FM, Zeng FQ, Wang L, Dong JH, Lu GC. Selection of optimal antisense accessible sites of survivin and its application in treatment of gastric cancer. World J Gastroenterol. 2005;11:634-640. [PubMed] |
| 2. | Schaft N, Dörrie J, Thumann P, Beck VE, Müller I, Schultz ES, Kämpgen E, Dieckmann D, Schuler G. Generation of an optimized polyvalent monocyte-derived dendritic cell vaccine by transfecting defined RNAs after rather than before maturation. J Immunol. 2005;174:3087-3097. [PubMed] |
| 3. | Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Howard CJ, Hope JC, Stephens SA, Gliddon DR, Brooke GP. Co-stimulation and modulation of the ensuing immune response. Vet Immunol Immunopathol. 2002;87:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Tsang KY, Zhu M, Even J, Gulley J, Arlen P, Schlom J. The infection of human dendritic cells with recombinant avipox vectors expressing a costimulatory molecule transgene (CD80) to enhance the activation of antigen-specific cytolytic T cells. Cancer Res. 2001;61:7568-7576. [PubMed] |
| 6. | Eppler E, Hörig H, Kaufman HL, Groscurth P, Filgueira L. Carcinoembryonic antigen (CEA) presentation and specific T cell-priming by human dendritic cells transfected with CEA-mRNA. Eur J Cancer. 2002;38:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Grégoire M, Ligeza-Poisson C, Juge-Morineau N, Spisek R. Anti-cancer therapy using dendritic cells and apoptotic tumour cells: pre-clinical data in human mesothelioma and acute myeloid leukaemia. Vaccine. 2003;21:791-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Engeland WC, Ennen WB, Elayaperumal A, Durand DA, Levay-Young BK. Zone-specific cell proliferation during compensatory adrenal growth in rats. Am J Physiol Endocrinol Metab. 2005;288:E298-E306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Orentas RJ, Schauer D, Bin Q, Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol. 2001;213:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Schjetne KW, Thompson KM, Aarvak T, Fleckenstein B, Sollid LM, Bogen B. A mouse C kappa-specific T cell clone indicates that DC-SIGN is an efficient target for antibody-mediated delivery of T cell epitopes for MHC class II presentation. Int Immunol. 2002;14:1423-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Liu Y, Zhang W, Chan T, Saxena A, Xiang J. Engineered fusion hybrid vaccine of IL-4 gene-modified myeloma and relative mature dendritic cells enhances antitumor immunity. Leuk Res. 2002;26:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Baxevanis CN, Voutsas IF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M. Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol. 2000;164:3902-3912. [PubMed] |
| 13. | Klinman DM, Sechler JM, Conover J, Gu M, Rosenberg AS. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388-2392. [PubMed] |
| 14. | Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102-3108. [PubMed] |
| 15. | Dorothee G, Ameyar M, Bettaieb A, Vergnon I, Echchakir H, Bouziane M, Chouaib S, Mami-Chouaib F. Role of Fas and granule exocytosis pathways in tumor-infiltrating T lymphocyte-induced apoptosis of autologous human lung-carcinoma cells. Int J Cancer. 2001;91:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | You Z, Huang XF, Hester J, Rollins L, Rooney C, Chen SY. Induction of vigorous helper and cytotoxic T cell as well as B cell responses by dendritic cells expressing a modified antigen targeting receptor-mediated internalization pathway. J Immunol. 2000;165:4581-4591. [PubMed] |
| 17. | Kugler A, Stuhler G, Walden P, Zöller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Müller CA, Becker V. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 417] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Steinwaerder DS, Carlson CA, Otto DL, Li ZY, Ni S, Lieber A. Tumor-specific gene expression in hepatic metastases by a replication-activated adenovirus vector. Nat Med. 2001;7:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |