INTRODUCTION
Gallbladder carcinoma is the fifth most common malignancy of the digestive tract. Recent molecular genetic studies have shown that selected proto-oncogenes and tumor-suppressor genes are involved in the development and progression of gallbladder carcinoma, and a different spectrum of molecular genetic changes appears to be responsible for each of the different preneoplastic conditions[1]. An association of gallbladder carcinoma with cholelithiasis or an anomalous arrangement of the pancreaticobiliary duct suggests that long-term inflammation may modulate tumorigenesis as well as the progression of this type of carcinoma[2,3]. Based on the histopathological examination of surgically resected gallbladder, an assumption has been made that epithelial changes found adjacent to invasive carcinomas in the gallbladder wall are premalignant and may progress to carcinoma. The rarity of most presumptive premalignant conditions has made it difficult to obtain better knowledge of the biological characteristics of precursor lesions of gallbladder carcinoma. At present, three major putative pathways of gallbladder carcinogenesis seem to be important: dysplasia, adenoma and anomalous pancreatico-biliary ductal junction. Roa[4] suggested that the period required to transform dysplasia into an advanced carcinoma is about 15 years. However, gallbladder carcinomas are usually diagnosed at an advanced stage, representing a challenge in the management because they respond poorly to therapy and are therefore associated with a poor prognosis. A postoperative 5-year survival rate between 5% and 13% has recently been reported[5,6]. Since this type of cancer is so difficult to cure by surgery alone, new molecular targets are needed for its prevention and treatment.
Prostaglandins (PGs), a family of lipid-derived autocrine and paracrine mediators, can favor tumorigenesis by altering cell proliferation, differentiation and adhesion, such as by modulating vascular response and immune surveillance. PGs are derived from arachidonic acid. The key step in the conversion of free arachidonic acid to PG is catalysed by the cyclooxygenase enzyme (COX) [7]. Three isoforms of COX (COX-1, COX-2 and COX-3) have been identified. While COX-1 is constitutively expressed as a housekeeping gene in most cells, COX-2 is not detected in normal tissues, except in kidney, liver and pancreatic islands, although it is expressed as an early response to many stimuli, such as inflammatory cytokines, growth factors and oncogens[8]. There is only little information about the recently identified COX-3 isoform. PGs have been shown to mediate gallbladder inflammatory response, since non-steroidal anti-inflammatory drugs (NSAIDs) have been proved to decrease gallbladder inflammation[9]. Accumulating evidences suggest that increased PGs levels via overexpression of the inducible COX-2 isoform are important in the development of human cancer[10]. Though increased levels of PGs and COX-2 expression have frequently been found in malignant growths of the digestive tract (colon, stomach, oesophageal cancer) similar data about biliary tumors are rare[11,12].
Point mutations or deletions of the p53 gene are observed in approximately 50% of cancers. Wild-type p53 is a key molecule in the cellular DNA damage response, causing restriction of cell proliferation by inducing cell cycle arrest and apoptosis. The product of mutant p53 has often been found to be a stable protein with a long half-life, while wild-type p53 has a short half-life and is not generally accumulated to a sufficient high level to be detectable by standard immunohistochemistry. An increase in the p53 protein level can therefore be used as an indication of p53 mutation, although there are still some discrepancies[13]. Immunohistochemically, p53 accumulation was detected in 36%-67.8% of gallbladder carcinomas[14,15]. In gallbladder carcinogenesis, an accumulation of p53 protein was reported in association with premalignant transition to malignancy[16]. p53 and COX-2 are thus two important molecules intimately linked to tumorigenesis. COX-2 has been implicated in positive regulation of growth and tumorigenesis, while the tumor suppressor p53 is a negative regulator of these processes. However, there is evidence of COX-2 expression normally suppressed by wild-type p53, suggesting that loss of p53 function may influence COX-2 overexpression[17]. Conversely, COX-2 can in turn inhibit p53-dependent transcription[18].
The purpose of this study was to evaluate the relationship and potential role of COX-2 and p53 in gallbladder carcinogenesis. Immunohistochemically, we analysed the expression profile of COX-2 in gallbladder dysplasia and carcinoma and its correlation with p53 protein accumulation.
MATERIALS AND METHODS
Subjects
A retrospective analysis was performed on 68 gallbladder tissue specimens, including 14 normal epithelium, 11 low-grade dysplasia, 16 high-grade dysplasia and 27 adenocarcinomas, obtained from patients treated with cholecystectomy in the period from 1998 to 2000. No patient had a history of regular ingestion of selected drugs such as NSAIDs or COX-2 inhibitors. We studied 14 cases of normal gallbladder epithelium without dysplastic and/or carcinomatous epithelial changes and 11 with low-grade dysplasia from elective cholecystectomy because of gallstones. In addition, 16 samples of high-grade dysplasia and 27 adenocarcinoma samples were obtained from patients who underwent cholecystectomy due to gallbladder adenocarcinoma. Dysplasia was defined histologically by varying degrees of pseudostratification, nuclear atypias, loss of polarity, and mitotic figures. Depending on the severity of changes, dysplasia was divided into low- and high-grade types. Adenocarcinomas were graded according to the World Health Organisation classification of gallbladder tumors[19]. There were 8 well differentiated, 4 moderately differentiated and 15 poorly differentiated adenocarcinomas.
Methods
COX-2 and p53 expression were investigated by immunohistochemistry. Formalin-fixed, paraffin embedded specimens were sectioned in series at a thickness of 5 μm. After deparafinisation, the slides were immersed for 30 min in 0.3% hydrogen peroxide/methanol to deplete endogenous peroxidase. Antigen retrieval was achieved using the pressure-cooking method for 4 min in citrate buffer (pH = 6.0). For COX-2 expression, polyclonal anti-human COX-2 antibody (Cayman Chemical Company, Ann Arbor, MI, USA) was used in a dilution of 1:500. For p53 analysis, monoclonal mouse anti-human p53 antibody, DO-7 (DAKO, Glostrup, Denmark) diluted at 1:100 was used. After the application of primary antibodies, the specimens were treated with biotinylated secondary antibody for 30 min. Antigen visualisation was achieved by applying a standard streptavidin-biotin complex (ABC, DAKO, Denmark) for 30 min followed by diaminobenzidin chromogen (DAB, Sigma Chemical CO, Germany) in 0.1% H2O2 PBS solution. The specimens were counterstained with haematoxylin. The specificity of the applied antibodies was checked with positive or negative controls. Specimens treated without primary antibodies served as negative controls. For positive controls of p53, specimens of human colon carcinoma with known p53 immunoreactivity were processed in the same way as the gallbladder samples. Vascular endothelial cells and fibroblasts observed in all gallbladder specimens provided internal positive controls for COX-2 staining.
Immunostaining was independently evaluated by two investigators (AC and ML) and a consensus agreement was achieved. COX-2 expression was evaluated according to the percentage of positive cells and the intensity of staining. The percentage of positive cells was scored as 0 (0%), 1 (< 10%), 2 (10-50%), 3 (51%-80%) or 4 (> 80%). The staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate) or 3 (strong). For immunoreactive score (IRS), the percentage of positive cells and the staining intensity were multiplied, resulting in a value between 0 and 12. To separate tumors with weak from those with strong COX-2 expression and to define a cut-off point that might be reproducible for future studies, we united samples with an IRS of 0-6 into one group with negative to weak COX-2 expression (the COX-2 negative group) and those with an IRS of 7-12 (the COX-2 positive group). The minimum requirement for positive IRS was therefore either moderate expression in > 80% of cells or strong expression in > 50% of cells. The expression of p53 was evaluated according to the percentage of positive cell nuclei. The reaction to p53 was considered positive when at least 20% of the nuclei in tumor cells were stained.
Statistical analysis
SPSS 11.0 for Windows (SPSS Inc., Chicago IL) was used. Chi-square tests were used to analyse the correlation between COX-2 overexpression and p53 accumulation and pathological features. The relationship between COX-2 and p53 expression was evaluated by the Mann-Whitney U-test. P < 0.05 was considered statistically significant.
RESULTS
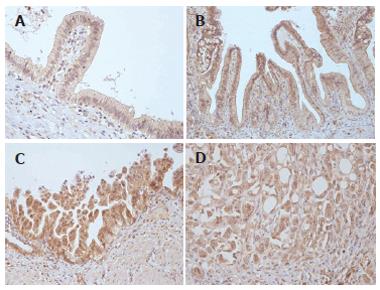
Surgical specimens of the gallbladder were obtained from 68 patients who had undergone cholecystectomy for clinical chronic cholecystitis or cholelithiasis (n = 51) and primary gallbladder carcinoma (n = 27). There were 43 female and 25 male patients, the median age of patients was 59 years (range from 36 to 78 years). The expression of COX-2 and p53 was analysed by immnohistochemistry in 14 cases of normal gallbladder epithelium, 27 cases of dysplasia and 27 adenocarcinomas. The cytoplasm of gallbladder epithelial cells was stained for COX-2 to various degrees (Figure 1). Additionally, COX-2 immunoreactivity was observed in fibroblasts, endothelial cells, and smooth muscle cells in all specimens. Normal gallbladder epithelium was COX-2 positive (according to the cut-off criteria of the IRS index) in only 2 (14.3%) cases. Of 27 cases of dysplasia, 19 (70.3 %) were COX-2 positive. When we divided this group into low- and high-grade dysplasia, COX-2 was positive in 5 (45.4%) cases of low-grade dysplasia and 14 (87.5%) cases of high-grade dysplasia (P = 0.019). The differences between normal epithelium and low-grade dysplasia were not statistically significant (P = 0.085). COX-2 was positive in 16 (59.2%) of 27 carcinomas.
Figure 1 COX-2 expression.
A: Normal gallbladder epithelium; B: Low-grade dysplasia; C: High-grade dysplasia; D: Gallbladder adenocarcinoma.
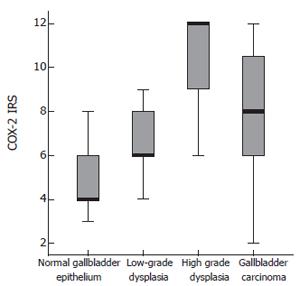
The greatest positivity for COX-2 was in poorly differentiated carcinomas (73.3%). The differences between high-grade dysplasia and carcinoma were not statistically significant (P = 0.113). COX-2-IRS values detected in normal gallbladder epithelium, dysplasia and carcinoma are shown in Figure 2.
Figure 2 COX-2 – IRS index.
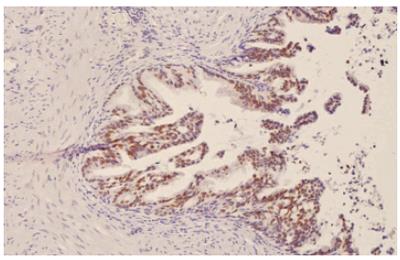
p53 was negative in normal gallbladder epithelium and low-grade dysplasia,. Accumulation of p53 protein was identified in the nuclei of 5 (31.2%) cases of high-grade dysplasia (Figure 3) and 13 (48.1%) carcinomas. Accumulation of p53 protein was the highest (80%) in poorly differentiated gallbladder carcinoma. In the whole spectrum of 68 tissue samples, we found only one COX-2 negative case among 18 p53 positive cases. Of 50 p53 negative cases, 30 (60 %) cases were COX-2 negative (P < 0.01).
Figure 3 Accumulation of p53 in nuclei of high-grade gallbladder dysplasia.
DISCUSSION
Our analysis of COX-2 and p53 expression in normal, dysplastic (premalignant) and malignant gallbladder epithelium is an attempt to reveal a possible relationship between the two molecules, as well as their role in gallbladder carcinogenesis. The role of COX enzymes in the process of carcinogenesis has been dramatically emphasized by confirmation that NSAIDs decrease the number of colonic polyps in humans with adenomatous polyposis and lower the incidence of colorectal cancer[20]. Gallbladder cancer has several analogous characteristics with this type of neoplasia. It is preceeded by a lengthy precancerous process taking several years. This process is characterized by chronic inflammation, lithyasis, intestinal metaplasia, and dysplasia in the epithelium of the surrounding mucosa[4]. We observed overexpression of COX-2 in 14 (87.5%) of 16 gallbladder high-grade dysplastic tissues in comparison to 5 out of 11 (45.4%) low-grade dysplasia and 2 (14.3%) of 14 normal gallbladder epithelium. Some slight COX-2 immunoreactivity in normal gallbladder epithelium was not unexpected and could be explained by the well-established connection between COX-2 expression and inflammation[20]. In carcinomas, COX-2 overexpression was found in 16 (59.2%) of 27 gallbladder samples. Our results are consistent with studies by Asano et al[11] and Zhi et al[21], who found 67.3% and 71.9% gallbladder tumour samples with COX-2 overexpression, respectively. Recently, Tsuchida[3] reported that COX-2 inhibitor meloxicam suppresses carcinogenesis in the gallbladder in an animal model. Our observation that COX-2 overexpression was highest in high-grade dysplasia supports the hypothesis of the role of COX-2 in early carcinogenesis. Liang[22] first suggested that COX-2 expression might be involved in the early proliferative phase but not in the late metastatic phase of the evolution of colorectal cancer. Our study is in agreement with Luzar[23] , who distinguished three histological degrees of gallbladder epithelial changes according to the number of hTERT signals: a) normal and regenerative gallbladder mucosa, b) low-grade dysplasia and c) high-grade dysplasia and adenocarcinoma of the gallbladder. We found no statistical differences in our study in COX-2 expression between normal gallbladder epithelium and low-grade dysplasia (P = 0.085), probably due to COX-2 expressed in normal mucosa on account of inflammation. However, the differences were significant between low-grade and high-grade dysplasia. No direct data are yet available about the progression rate of high-grade dysplasia to invasive carcinoma of the gallbladder. For comparison, high-grade dysplasia in the stomach was detected to regress in only 5% of cases, persisted in 14%, and progressed in 81%-85%[24] .
In high-grade dysplasia, p53 protein accumulation was found in 5 (31.2%) cases. Low-grade dysplastic and normal epithelium was p53 negative. Thirteen (48.1%) of 27 carcinomas were p53 positive. The clone against which the p53 antibody was raised (DO-7) results from the recognition of wild type and mutated protein. However, p53 detection was not expected in normal cells. The appearance of a nuclear accumulation of p53 protein might arise from mutation of the protein itself, resulting in failure of its degradation pathway or a mutation of the human mouse double minute (hMDM)-ubiquitin pathway, resulting from the failure of degradation. Accumulation of the wild-type p53 protein owing to a failure of degradation might push the cell into cell cycle arrest and apoptosis. Such cells would occur rather sparsely in tumors because they could not proliferate as malignant clones. It therefore seems very likely that the accumulation of p53 in dysplastic and malignant gallbladder epithelium resulted from p53 mutation[24]. Our results are consistent with some previous reports about p53 gene mutations involved in the carcinogenesis of the gallbladder epithelium[26,27] that p53 overexpression appeard in only 84.6% of gallbladder carcinomas and in 18.7% of patients without carcinoma. Matsubara[28] detected p53 gene mutation in 38.5% of noncancerous biliary epithelial lesions and in 57.1% of adenocarcinomas.
Data about the inter-relationship between p53 and COX-2 are conflicting. Our results are consistent with studies of Shigemasa[29] and Erkinheimo[30]. In both their studies, performed on ovarian carcinoma, a significant positive correlation was found between COX-2 expression and p53 accumulation. The same correlation was accepted in a study of head and neck tumors[31], suggesting that restoration of wild-type p53 expression might interfere with tumour growth by inhibiting the COX-2 pathway. In endometrial carcinoma[32] and colon carcinoma[22], higher COX-2 expression was also associated with p53 accumulation. In contrast, Cho[33] observed no association between COX-2 overexpression and p53 accumulation in renal cell carcinoma. Subbaramaiah[34] demonstrated that wild-type p53 suppressed COX-2 expression by inhibiting its promotor activity. The level of COX-2 protein and mRNA was found markedly decreased in cells expressing wild-type, but not mutant p53[35]. A study of head and neck carcinoma cell lines[36] confirmed that wild-type p53 overexpression has some anti-tumor effects through the suppression of COX-2 gene activity. However, Swamy[18] reported that COX-2 at least in part contributes to the dysfunction of p53 and that celecoxib, a COX-2 selective inhibitor, protects p53 functional activity.
In conclusion, our study is the first to analyse the relationship of COX-2 expression and p53 accumulation in various histological stages of gallbladder epithelial abnormalities and gallbladder adenocarcinoma. Our results suggest that COX-2 might play an important role in gallbladder carcinogenesis. Overexpression of COX-2 appears to be an early event in gallbladder carcinogenesis, already occurring at the stage of preneoplastic epithelial changes (e.g. high-grade dysplasia) and may be, at least in part, related to p53 dysfunction.
S- Editor Pan BR L- Editor Ma JY E- Editor Bi L