INTRODUCTION
Obesity is currently a serious medical problem world wide. It is an independent risk factor of non-alcoholic steatohepatitis (NASH) and alcoholic liver injury[1,2]. The pathogenesis of these diseases involves lipopolysaccharide (LPS)[3-5]. The complex of LPS and LPS-binding protein (LBP) activates Kupffer cells to secrete tumor necrosis factor (TNF)-α, which plays an important role in liver injury[6,7]. The control of the response of Kupffer cells to LPS is thought to be critical in the prevention of LPS-induced liver injury. Indeed, the selective inhibition of Kupffer cells by the administration of either gadolinium chloride or methyl palminate results in the abrogation of liver injury with an inhibition in TNF-α secretion[8]. Interleukin (IL)-10, which is secreted by Kupffer cells in the liver after LPS stimulation[9], has strong anti-infla-mmatory effects in the liver, and prevents liver fibrosis[10,11]. Interferon (IFN)-γ activates the signal transducer and activator of transcription (STAT-1) in hepatocytes and promotes apoptosis in hepatocytes after administration of D-Galactosamine (GalN)/LPS[12]. In GalN/LPS induced liver injury, the modulation of the production of these cytokines from Kupffer cells is important.
Adiponectin, a 30 ku adipocyte complement-related protein (Acrp30), is an adipocyte-specific plasma protein (normal level, 5-30 mg/L), and is paradoxically decreased in obesity and in cases of alcoholic fatty liver[13,14]. Adiponectin contains two types of receptors, AdipoR1 and AdipoR2. Whereas AdipoR1 is expressed ubiquitously and abundantly in skeletal muscle, AdipoR2 is most abundantly expressed in the liver and is an intermediate-affinity receptor for full-length and globular adiponectin[15]. Adiponectin improves the insulin sensitivity, and has anti-atherogenic and anti-inflammatory effects. In the liver, it has anti-fibrogenic effects and regulates hepatic stellate cells[16].
We recently reported that adiponectin inhibits the phagocytosis of human macrophages and LPS-induced TNF-α release[17], and induces IL-10 gene expression in human macrophages[18]. We hypothesized that adiponectin has anti-inflammatory effects on Kupffer cells, key modulators of LPS induced liver injury, by regulating the release of cytokines. However, the effects of adiponectin on LPS-induced liver injury and Kupffer cells remained poorly understood. In the present study, to clarify the effects of adiponectin on LPS-induced liver injury, we investigated the effects of adiponectin on GalN/LPS-induced liver injury using adiponectin knockout mice. We also examined the effects of adiponectin on cytokine production from Kupffer cells in primary cultures.
MATERIALS AND METHODS
All experimental protocols described in this study were approved by the Ethics Review Committee for Animal Experimentation of Osaka University School of Medicine.Recombinant mouse full-length adiponectin was prepared as described previously[16]. The method of disruption of the mouse adiponectin gene was described previously[19].
Murine model of acute hepatitis
To clarify the role of adiponectin in LPS-induced liver injury, experiments were conducted using adiponectin-/- mice and wild-type (WT) control C57B6 mice (28-32 g body mass; 10-12 wk old)[19]. Each mouse was simultaneously injected intraperitoneally with 700 mg/kg of GalN and 10 μg/kg of LPS. The doses of GalN and LPS were determined in previous studies and our preliminary study[12]. LPS from E. coli O55:B5 and D-galactosamine (GalN) were purchased from Sigma (St. Louis, MO). Groups of 10 mice were treated to determine the survival curve. Groups of 6 mice were treated to measure the plasma levels of alanine aminotransferase (ALT), TNF-α, IL-10, and IFN-γ. Groups of 6 mice were sacrificed as follows: before, 0.5, 1, and 4 h after the administration to measure the gene expressions of TNF-α IL-10, IFN-γ in the whole liver and IFN-γ in the spleen, assessed by means of real-time polymerase chain reaction (PCR).
LPS stimulation on Kupffer cells
Male Sprague-Dawley (SD) rats (10 wk old, n = 6) were anesthetized with pentobarbital sodium, and the portal vein was cannulated. The liver was perfused with a ethylene glycol bis (beta-aminoethyl ether)-N, N, N’, N’-tetraacetic acid (EGTA) solution, and digested with a 0.5 g/L collagenase solution. Differential centrifugation on Nycodenz (Pharma, Oslo, Norway) density gradients was performed as described before[20]. We evaluated the purity of the isolated Kupffer cell population by counting CD68 positive cells, ranging from 88.5% to 93.0%. Kupffer cells from each rat were maintained and treated separately. The Kupffer cells were maintained at 37˚C under 50 mL/L CO2 in Dulbecco’s modified Eagle medium containing 100 mL/L fetal calf serum with or without adiponectin (10 mg/L) for 24 h. After a 24 h pretreatment with and without adiponectin, LPS was added to the culture medium at a concentration of 10 mg/L. The doses of adiponectin and LPS were determined in previous studies and our preliminary study[6,17]. TNF-α, IL-10, IFN-γ concentrations in the culture medium were measured 0 and 4 h after the commencement of LPS stimulation. Total RNA of Kupffer cells was isolated at 0, 0.5, 1, and 4 h after the commencement of LPS stimulation.
Measurement of plasma concentrations of ALT and cytokines
Plasma ALT concentrations were measured by using a transaminase CII-test kit according to the protocol provided by the manufacturer (Wako Pure Medical, Osaka, Japan). Circulating levels of TNF-α, IL-10, and IFN-γ were assessed using commercial enzyme-linked immunosorbent assay (ELISA) kits for mice (Biosource Int., Camarillo, CA). Their concentrations in the culture media of Kupffer cells were quantified using ELISA kits for the rat (Biosource Int.).
Quantification of gene expression levels
Total RNA from whole liver, spleen and Kupffer cells were extracted using a Qiagen (Hilden, Germany) QIAshredder and an RNA-easy Mini kit according to the instructions provided by the manufacturer. The reverse-transcription polymerase chain reaction (RT-PCR) was performed as described previously[21]. The Quantitect PCR probe kit and Quantitect gene assay kit for mice TNF-α, IL-10, and IFN-γ were purchased from Qiagen and used in a real-time PCR analysis of the mouse samples. Primers for rat glyceraldehydes-3-phosphate dehydrogenase (GAPDH), TNF-α, IL-10, IFN-γ, and mice GAPDH were designed using the computer program Primer Express (Applied Biosystems, Foster city, CA). Dynamo SYBR Green qPCR kit (Finzymes, Espoo, Finland) was used for the real-time PCR analysis of rat TNF-α, IL-10, IFN-γ, GAPDH and mice GAPDH. Real-time PCR was performed using a DNA Engine Opticon 2 real-time PCR Detection System (MJ Research, Waltham, MA). Relative gene expression was quantified using GAPDH as an internal control. The expressions of adiponectin receptors, AdipoR1 and AdipoR2 were assessed with RT-PCR as previously published[22].
TUNEL assay
Quantification of apoptotic hepatocytes in liver sections was performed by counting the number of TUNEL-positive cells. The data were expressed as the average number of TUNEL-positive cells per five high power fields (HPF) (×100). The TUNEL assay was performed based on the instructions provided by the manufacturer (In Situ Apoptosis Detection Kit; TaKaRa, Shiga, Japan, and Liquid DAB Substrate kit; Zymed Lab. Inc., South San Francisco, CA).
Statistical analysis
The results are presented as the mean ± SE. Analysis of variance (ANOVA) for the groups was performed by the Mann Whitney test, followed by Scheffé’s test for multiple comparisons to allow pairwise test for significant differences between groups. The statistical significance of the lethality rates was determined by a Log-rank test. Statistical significance was defined as P < 0.05.
RESULTS
GalN/LPS induces severe liver injury in KO mice
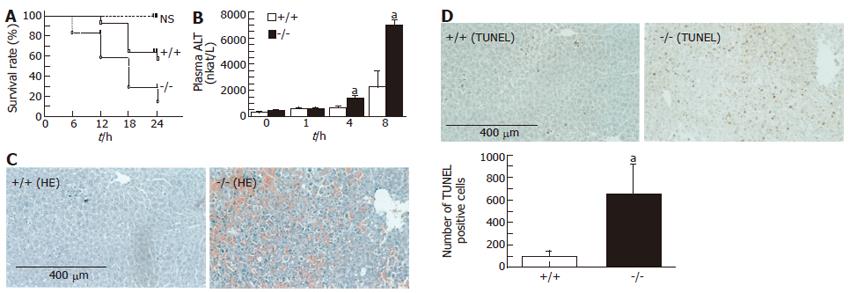
The survival rate of adiponectin-/- mice after GalN/LPS administration was significantly lower than that of WT mice 24 h after the administration (P = 0.041; log-lank test; Figure 1A). GalN/LPS administration resulted in an elevation of plasma ALT concentrations in both WT and adiponectin-/- mice, although increases at 4 and 8 h after administration were significantly higher in adiponectin-/- mice than in WT mouse (at 4 h; WT, 693.5 ± 472.8 vs adiponectin-/-, 1458.6 ± 541.8 nkat/L; P < 0.01, at 8 h; WT, 2255.5±3242.3 vs adiponectin-/-, 6988.1 ± 978.5; P < 0.05; Figure 1B). A histological examination revealed that GalN/LPS administration induced massive liver injury in adiponectin-/- mice mice (Figure 1C). The number of TUNEL-positive hepatocytes in adiponectin-/- mice livers was significantly higher than that in WT mouse livers 8 h after GalN/LPS administration (WT, 95 ± 118 vs adiponectin-/- mice, 683 ± 729 apoptotic cells/HPF; P < 0.05; Figure 1D).
Figure 1 GalN/LPS induced changes in mice.
A: Survival rate (n = 10); B: Plasma ALT (mean ± SE, n = 6). aP < 0.05, bP < 0.01, vs WT; C: Histology of the liver. Massive liver injury in adiponectin-/- mice. (Hematoxylin-eosin × 100); D: Large numbers of apoptotic hepatocytes in adiponectin-/- mice (TUNEL × 100). Scale bar = 400 μm. aP < 0.05, vs WT mice.
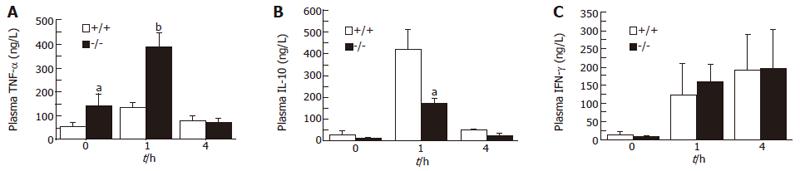
Effects of GalN/LPS on plasma TNF-α, IL-10 and IFN-γ
Prior to GalN/LPS administration, plasma TNF-α concentrations were significantly higher in adiponectin-/- mice than in WT mice (P < 0.05; Figure 2A). Plasma TNF-α concentrations increased reaching peak levels 1 h after the administration, and were significantly higher in adiponectin-/- mice mice than in WT mice at their peak (P < 0.01; Figure 2A). Plasma IL-10 concentrations also increased, reaching peak levels 1 h after the administration of GalN/LPS. In adiponectin-/- mice, IL-10 plasma concentrations were significantly lower than those of WT mice at their peak (P < 0.05; Figure 2B). Plasma IFN-γ concentrations increased continuously up to 4 h after GalN/LPS administration, and no significant difference between adiponectin-/- mice and WT mice was found (Figure 2C).
Figure 2 Plasma concentrations of TNF-α, IL-10 and IFN-γ in mice after GalN/LPS administration.
(mean ± SE, n = 6). aP < 0.05, bP < 0.01, vs WT.
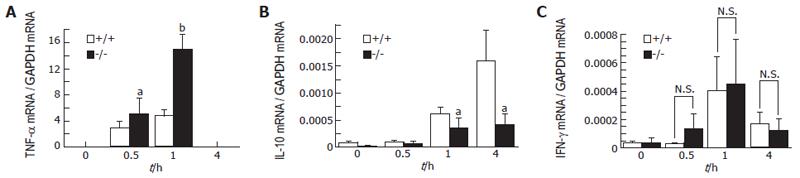
Effects of GalN/LPS on liver TNF-α, IL-10, IFN-γ and splenic IFN-γ mRNA expression
The TNF-α mRNA expression level in the liver increased, reaching a peak level 1 h after GalN/LPS administration, and this level was significantly higher in adiponectin-/- mice than in WT mice 1 h after the administration (P < 0.01; Figure 3A). The IL-10 mRNA expression level in the liver also increased, reaching a peak level 4 h after GalN/LPS administration, and the levels at 1 and 4 h were significantly higher in WT mice than in adiponectin-/- mice (at 1 h; P < 0.05; Figure 3B). The IFN-γ mRNA expression level in the liver increased, reaching peak levels 1 h after GalN/LPS administration, but no significant difference between adiponectin-/- mice and WT mice was found (Figure 3C). In the spleen, the IFN-γ mRNA expression levels also reached peak levels at 1 h after the administration, but there was no significant difference between adiponectin-/- mice and WT mice (data not shown).
Figure 3 TNF-α, IL-10 and IFN-γ in the liver (A-C) of mice following GalN/LPS administration.
(mean ± SE, n = 6). aP < 0.05, bP < 0.01, vs WT (Scheffé’s test).
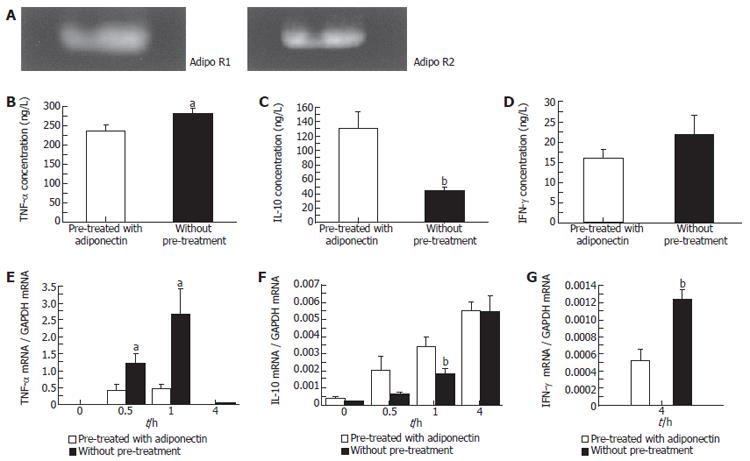
Adiponectin receptors expression in Kupffer cell
We comfirmed the expressions of AdipoR1 and AdipoR2 in rat Kupffer cells using RT-PCR (Figure 4A).
Figure 4 The expressions of adipoR1 and AdipoR2 in Kupffer cells (A).
Effect of adiponectin on TNF-α, IL-10 and IFN-γ production by LPS-stimulated Kupffer cells in vitro (B-D). Pre-treated with or without adiponectin (10 mg/L) for 24 h followed by the stimulation with LPS (10 mg/L). TNF-α, IL-10 and IFN-γ mRNA expression in Kupffer cells after LPS stimulation (E-G). (mean ± SE, n = 6). aP < 0.05, bP < 0.01 (Scheffé’s test).
Adiponectin inhibits LPS-induced TNF-α production in Kupffer cells
Pretreatment of Kupffer cells with adiponectin led to a reduction in the levels of TNF-α released in response to LPS stimulation. TNF-α levels in the culture media of adiponectin-pretreated Kupffer cells were significantly lower than those for untreated cells 4 h after LPS stimulation (P < 0.05; Figure 4B). The LPS-induced TNF-α mRNA expression level in Kupffer cells was markedly suppressed by adiponectin pre-treatment. The expression level of TNF-α mRNA in Kupffer cells increased, reaching peak levels 1 h after LPS stimulation, but the levels at 0.5 and 1 h after the commencement of LPS stimulation in culture media of adiponectin-pretreated Kupffer cells was significantly lower than in those of untreated cells (at 0.5 h; P <0.05, at 1 h; P < 0.05; Figure 4E).
Adiponectin increases LPS-induced IL-10 production in Kupffer cells
Pretreatment of Kupffer cells with adiponectin increased the release of IL-10 in response to LPS stimulation. IL-10 concentrations in the culture media of adiponectin-pretreated Kupffer cells were significantly higher at 4 h after LPS stimulation than those without adiponectin pre-treatment (P < 0.01; Figure 4C). The expression level of LPS-induced IL-10 mRNA in Kupffer cells was markedly increased as a result of adiponectin pre-treatment. The expression level of IL-10 mRNA in Kupffer cells was significantly higher in adiponectin-pretreated Kupffer cells 1 h after LPS stimulation than in untreated cells (P < 0.05; Figure 4F).
Lack of effect of adiponectin on LPS-induced IFN-γ production in Kupffer cells
Pretreatment of Kupffer cells with adiponectin had no significant effect on IFN-γ concentrations in the culture media (Figure 4D). But the expression level of LPS-induced IFN-γ mRNA in Kupffer cells was significantly higher in adiponectin-pretreated Kupffer cells 4 h after LPS stimulation than in untreated cells. IFN-γ gene expression in Kupffer cells at 0 and 1h after LPS stimulation could not be determined quantitatively by RT-PCR (P < 0.01; Figure 4G).
DISCUSSION
The major findings of the present study were that a lack of adiponectin accelerates LPS-induced liver injury, and that adiponectin has an anti-inflammatory effect on Kupffer cells. Our results demonstrate that adiponectin-pretreated Kupffer cells produced less TNF-α and more IL-10 as a result of LPS treatment. These results suggest that adiponectin has an anti-inflammatory effect on LPS-treated Kupffer cells. An intraperitoneal injection of GalN/LPS in mice resulted in a more serious liver injury that was associated with a significantly higher mortality in adiponectin-/- mice than in WT mice. The plasma levels of TNF-α were significantly increased and those of IL-10 were significantly decreased in adiponectin-/- mice compared to WT mice, and TNF-α gene expression in the liver was significantly higher and those for IL-10 were lower in adiponectin-/- mice than in WT mice. These results indicate that a lack of adiponectin enhances LPS-induced liver injury and that the altered production of cytokines in Kupffer cells caused by a lack of adiponectin affect the severity of LPS-induced liver injury.
TNF-α causes liver cell apoptosis in GalN-sensitized mice[7,23]. Mice that are deficient in TNF-α receptors are protected against GalN/LPS-induced liver damage[24]. In our study, plasma TNF-α levels increased significantly in adiponectin-/- mice after GalN/LPS administration compared with WT mice, suggesting that a lack of adiponectin causes an over response of TNF-α production. Indeed, TNF-α gene expression in the liver increased significantly in adiponectin-/- mice after GalN/LPS administration compared with WT mice. Sennello et al. recently reported that adiponectin protects hepatocytes from TNF-α induced cell death[25]. Our results are in agreement with this conclusion. We previously reported that, in peripheral macrophages, adiponectin inhibits LPS-induced TNF-α production possibly through the suppression of TNF-α-induced IκB-α-NF-κB activation via the cAMP-dependent pathway[17,26]. In the present study, adiponectin pre-treated Kupffer cells showed a lower response to LPS than the controls. The TNF-α concentration in the culture media of adiponectin pre-treated Kupffer cells showed a significantly lower elevation than those of untreated cells, and TNF-α gene expression levels after the LPS stimulation of adiponectin pre-treated Kupffer cells were significantly lower than those of untreated cells. The TLR4 receptor system plays an important role in innate immunity[27]. Kupffer cells of mice express TLR4 mRNA and respond to LPS. The LPS-binding protein (LBP) complex associates with the CD14, and via TLR4, activates Kupffer cells to secrete TNF-α[7]. Adiponectin may inhibit this receptor system directly by suppressing IκB-α-NF-κB activation, although further studies will be needed to confirm this conclusion.
IL-10 exhibits a hepatoprotective role in GalN/LPS-induced liver injury by inhibiting the release of TNF-α[10,11,28]. In our study, plasma IL-10 levels were significantly diminished in adiponectin-/- mice after the administration of GalN/LPS compared with WT mice. GalN/LPS treatment of adiponectin-/- mice significantly reduced IL-10 gene expression in the liver compared with WT mice. Kupffer cells are known to release substantial amounts of IL-10 following LPS stimulation[9]. In our study, IL-10 concentrations in the culture medium of adiponectin pre-treated Kupffer cells showed a significantly higher response to LPS stimulation than the control cells. We recently reported that adiponectin increases IL-10 gene expression in human macrophages[18]. IL-10 gene expression in adiponectin pre-treated Kupffer cells was also significantly higher than in untreated cells. These data show that physiological concentrations of adiponectin are sufficient to induce IL-10 production in Kupffer cells in response to LPS stimulation, and that a lack of adiponectin resulted in a lower IL-10 response to LPS stimulation in Kupffer cells. These results suggest that adiponectin inhibits TNF-α production directly as well as indirectly through the induction of IL-10. Further studies will be required to elucidate the precise mechanism of this cross-talk.
IFN-γ is also involved in the toxic effects of LPS on the liver[29], and IFN-γ monoclonal antibodies reduce LPS-induced mortality in mice[30]. The overexpression of IFN-γ activates STAT-1 in the liver and promotes hepatocyte apoptosis following GalN/LPS administration[12]. IL-10 is reported to inhibit IFN-γ[31]. However, Sennelo et al. recently reported that, in a model of Concavaline A induced hepatitis, there was no significant difference in serum IFN-γ levels between lipodystrophic aP2-nSREBP-1c transgenic mice, with higher serum adiponectin, and ob/ob mice, with lower serum adiponectin levels[25]. Our study found no significant differences in plasma levels of IFN-γ between adiponectin-/- mice and WT mice, or IFN-γ gene expression levels in the liver and splenic lymphocytes between adiponectin-/- mice and WT mice. We found no significant difference in IFN-γ concentration in the culture media of adiponectin pre-treated Kupffer cells and untreated Kupffer cells. However, 4 h after the LPS stimulation, IFN-γ gene expression levels of adiponectin pre-treated Kupffer cells were significantly lower than those of untreated cells. Further studies will be required to elucidate the effect of adiponectin on IFN-γ production in Kupffer cells.
Obesity is an independent risk factor for the development of chronic liver diseases such as NASH and alcoholic liver disease[1,2]. Serum adiponectin levels are decreased in obesity, and alcohol decreases the expression of adiponectin[13,14]. Adiponectin has been reported to confer protective effects against alcoholic liver injury, and hypoadiponectinemia is involved in the progression of non-alcoholic fatty liver disease (NAFLD) to steatohepatitis[32,33]. Our results suggest that the hypoadiponectinemia in obesity induces an altered response of Kupffer cells to LPS, and is possibly involved in the development of alcoholic liver disease and NASH. Adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) have been cloned by expression cloning[15]. AdipoR1 is expressed abundantly in skeletal muscle, and is also expressed in the liver. AdipoR2 is predominantly expressed in the liver. Thakur V et al showed the expressions of adipoR1 and R2 in Kupffer cells and we also found these expressions in Kupffer cells[34]. Recently Wolf AM et al reported the up-regulation of adiponectin in liver in ConA mediated acute liver failure[35]. In GalN/LPS induced acute liver injury, the expressions of adiponectin in the liver could not be determined quantitatively by RT-PCR (data not shown). To elucidate the effect of adiponectin produced in the liver in this model, further studies will be needed. Masaki et al reported that adiponectin prevents LPS-induced hepatic injury in a KK-Ay obese mice model[36]. KK-Ay obese mice show not only hypoadiponectinemia but also hyperglycemia and insulin resistance. Hyperglycemia and hyperinsulinemia should have an effect on the response of inflammatory cytokines to endotoxemia[37]. We used adiponectin-/- mice to rule out the effects of such other factors. In this model, we were able to clarify the direct effect of adiponectin deficiency on LPS-induced liver injury in vivo.
In conclusion, a deficiency of adiponectin could enhance LPS-induced liver injury possibly through modulation of cytokine production by Kupffer cells. The development of adiponectin receptor agonists or molecules that can induce adiponectin secretion might be helpful in controlling LPS-related liver diseases such as NASH and alcoholic liver injury.