Published online Jun 7, 2006. doi: 10.3748/wjg.v12.i21.3297
Revised: January 28, 2006
Accepted: February 18, 2006
Published online: June 7, 2006
Receptor tyrosine kinases (RTKs) such as the epidermal growth factor receptor family participate in several steps of tumor formation including proliferation and metastatic spread. Several known RTKs are upregulated in gastric cancer being prime targets of a tailored therapy. Only preliminary data exist, however, on the use of the currently clinically available drugs such as trastuzumab, cetuximab, bevacizumab, gefitinib, erlotinib, and imatinib in the setting of gastric cancer. Preclinical data suggest a potential benefit of their use, especially in combination with “conventional” cytostatic therapy. This review summarizes the current knowledge about their use in cancer therapy as well as new approaches and drugs to optimize treatment success.
- Citation: Becker J, Müller-Tidow C, Serve H, Domschke W, Pohle T. Role of receptor tyrosine kinases in gastric cancer: New targets for a selective therapy. World J Gastroenterol 2006; 12(21): 3297-3305
- URL: https://www.wjgnet.com/1007-9327/full/v12/i21/3297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i21.3297
Gastric cancer is a common, but difficult to treat disease entity. Apart from potentially curative surgery, chemotherapy as well as radiochemotherapy may be applied, but do not cure the disease[1]. Thus, improvement of gastric cancer therapy will depend on novel therapeutic approaches. Receptor tyrosine kinases (RTKs) are a family of 56 proteins characterized by a transmembrane domain and a tyrosine kinase motif[2]. They function in cell signalling and transmit signals regulating growth, differentiation, adhesion, migration, and apoptosis[3]. Aberrant receptor tyrosine kinase activity was initially described in various epithelial cancers; nowadays, it is well accepted that receptor tyrosine kinases play an important role in almost all types of cancer[4,5]. The mutational activation and/or overexpression of receptor tyrosine kinases transforms cells and often plays a crucial role in the development of cancers.
For this reason, RTKs have become targets of immunological inhibitors, the best known being trastuzumab (Herceptin®, directed against the erb B2 receptor, also known as Her2/Neu oncogene) and cetuximab (Erbitux®, active against the epidermal growth factor receptor [EGFR]). In the case of EGFR, pharmacological inhibitors such as gefitinib (Iresssa®)directed at the kinase domain (EGFR-TKI) of the receptor have been developed; other pharmacological inhibitors of tyrosine kinases include imatinib (Gleevec®) which is licensed for the treatment of leukaemia and gastrointestinal stroma cell tumors (GIST).
As tailored concepts of cancer therapy evolve, RTKs offer prime targets for such an individualized approach to cancer treatment. This review examines the information available on this group of receptors, describes the most relevant subsets that have been found in case of gastric cancer, and summarizes data on the use of their inhibitors in clinical studies. In the end, we present new attempts to optimize the efficacy of already available compounds and promising new drug developments.
RTKs are membrane bound proteins consisting of a ligand-binding domain at the extracellular surface, a single transmembrane segment, and a cytoplasmic part harboring the protein kinase activity. With the exception of the insulin receptor family of RTKs, all known RTKs form monomers in the cell membrane. Ligand-induced dimerization, resulting in autophosphorylation of their cytoplasmic domains, is the major mode of activation of RTKs.
The known 56 RTKs are divided into 21 families with similar structure and the potential of intrafamilial dimerization; their classification has been reviewed by Robinson et al[2]. The best known examples are the epidermal growth factor receptor family (erb B1 to B4), the different vascular endothelial growth factor receptor (VEGFR) subtypes, the fibroblast growth factor (FGF) receptor family, and the platelet derived growth factor (PDGF) receptor family.
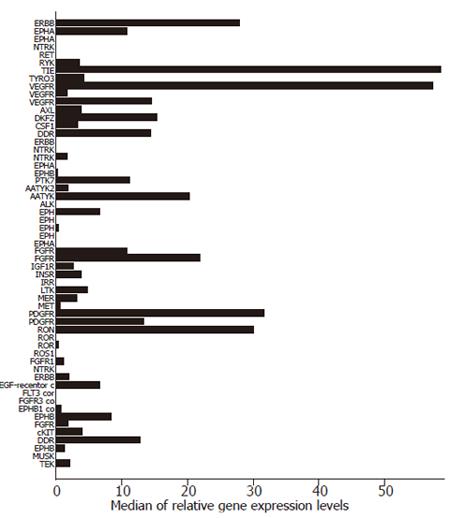
We have recently analyzed the expression of RTK mRNAs in different human cancers, including 12 samples of gastric cancer[6]. The median of relative gene expression levels of the 56 known RTKs in these cancer specimens are illustrated in Figure 1. Our findings suggest that several RTKs, including those of the EGFR family, the FGFR family, and the different VEGFR subtypes are present in gastric cancer and thus offer potential targets for a selective therapy. In the following, we will summarize findings by others regarding the expression of the RTKs in gastric cancer with emphasis on those for which clinically established modalities of therapeutic intervention exist, namely the EGFR family and VEGF.
The EGF receptor/ligand system seems to be involved in the regulation of gastric mucosal proliferation and progression of gastric carcinoma. Increased EGFR binding has been observed in gastric carcinomas in comparison to adjacent normal gastric mucosa. Moreover, elevated EGFR levels have been found in gastric carcinoma with worse prognostic factors (T3/4, positive lymph-nodes, G3, diffuse-type). In univariate and multivariate analysis, EGFR levels are an independent indicator of poor prognosis[7,8]. A down-regulation of an inhibitor of EGFR activation, EGFR related protein (ERP) occurs in gastric cancer as well[9]. Changes in the constitutive phosphorylation and activity of tyrosine kinases such as EGFR may contribute to differences in cell adhesion and phenotype of gastric cancer cells[10]. Espinoza et al described that gastric adenocarcinomas potentially depend upon the TGFalpha-EGFR autocrine loop for growth and exhibit increased aggressiveness in the presence of aberrant p53[11].
The overexpression of the growth factor receptor oncogene erb B2 in gastric carcinomas was first described by Yonemura et al in 1991, who reported a 12% positive rate in 189 gastric cancers[12]. These findings were confirmed by many other studies, which provided further evidence that the highest rate of overexpression was found in patients with advanced disease, and that erb B2 altered expression can be considered as an independent predictor of outcome[13,14]. Ougolkov et al[15] found a more frequent overexpression of erb B2 in gastric cancers with concurrent liver metastasis than in those without, and concluded that activated erb B2 may be involved in the process of liver metastasis thus suggesting a role for erb B2 overexpression in identifying gastric cancer patients who are at high risk of developing liver metastasis. In contrast, activating mutations of the erb B2 tyrosine kinase seem to be rare events in gastric cancer[16].
Vascular endothelial growth factor (VEGF) is expressed in many gastric carcinoma cell lines and may play an important role in cell growth directly. VEGFR is virtually ubiquitous in human tumors and may promote tumor growth and metastasis by participating in both paracrine and autocrine pathways as higher levels of VEGFR have been correlated with more aggressive disease[17]. KDR is the main human receptor responsible for the VEGF activity in both physiological and pathological vascular development, and VEGF-KDR signalling pathway has been validated as a priority target for the development of anti-cancer therapy. A first report on 16 patients with early gastric cancer (pT1-T2, N0) suggested that expression of VEGF may indicate an increased risk of recurrence, making VEGF neutralization an interesting therapeutic target in these subjects[18].
We have previously described an increased expression and autocrine stimulation of fibroblast growth factor (FGF) 2 mRNA in the course of gastric ulcer healing[19]; with respect to gastric cancer, the secretion of growth factors of the FGF family by fibroblasts stimulates proliferation of cancer cells of scirrhous gastric cancer[20]. The expression of FGF2 mRNA (formerly known as basic fibroblast growth factor, bFGF) in gastric cancer specimens is associated with higher microvascular density, tumor progression and a worse prognosis[21]. An orally active inhibitor of FGF-receptor autophosphorylation has been successfully tested in animal models of gastric cancer; further studies are awaited[22].
Already more than a decade ago, the expression of platelet derived growth factor (PDGF) and its receptor mRNAs were linked to gastric cancer growth[23]. Later on, the expression of PDGF-A mRNA in biopsy samples of gastric cancer specimens was determined to be a preoperative prognostic marker associated with shorter survival[24]. As imatinib mesylate as an inhibitor of the PDGFR tyrosine kinase became available, its effectiveness against gastric cancer cells was assessed in vitro and in vivo. Unfortunately, the agent itself exhibited no cytostatic effect of relevance; yet in combination, imatinib mesylate might serve as an effective chemosensitizer of antitumor drugs, such as 5-FU and paclitaxel for gastric carcinoma, targeting the PDGF/PDGFR-signalling pathway of tumor cells and stromal cells in disease progression and angiogenesis[25].
Leflunomide is a small molecule inhibitor of PDGFR-mediated phosphorylation and inhibits PDGF-mediated cell signalling[26]; it is converted to its main metabolite, SU0020, which interferes with de novo pyrimidine synthesis. At this time, it is not clear whether the mechanism of action of this drug in humans is due to inhibition of PDGF-dependent signalling, inhibition of pyrimidine synthesis, or a combination of both[27-29]. A multi-institutional phase II study in hormone refractory prostate cancer patients with leflunomide found partial responses in 1 of 19 patients, a prostate-specific antigen decline greater than 50% in 3 of 39 patients, and improvement in pain[27].
Soman et al were able to identify TPR-MET fusion as an event of gastric cancer pathogenesis in certain cases[30]; in addition, c-MET activation seemed to occur[31]. Preliminary data raised in animal models of gastric carcinoma suggest that adenoviral-mediated gene transduction of an HGF antagonist (NK4) results in suppression of tumor growth, invasion, angiogenesis, and metastasis[32,33]. The use of this construct was able to overcome resistance against gefitinib in an animal model of scirrhous gastric cancer[34], yet clinical data is lacking.
The inhibitors of tyrosine kinases currently approved by the United States Food and Drug Administration (FDA) for the use in different human malignancies are summarized in Table 1. Substances under development are described in the paragraphs below. If treatment results of RTKIs are to be evaluated, one has to keep in mind that inhibition of a growth factor receptor does not typically aim at killing the cell like “conventional” cytostatics, but stoping proliferation. Therefore, standard assessments of tumor response like complete response, partial response, and stable disease may not reflect the “true” efficacy of such a regime.
| Agent | Target | Mode of action | Established clinicalapplication |
| Imatinib mesylate | bcr-abl; PDGFR, c-kit | Kinase inhibitor | CML, GIST, hypereosinophilic syndrome |
| Gefitinib | erbB1/EGFR | Kinase inhibitor | NSCLC |
| Erlotinib | erbB1/EGFR | Kinase inhibitor | NSCLC |
| Cetuximab | erbB1/EGFR | Blocking antibody | Colorectal cancer |
| Trastuzumab | erbB2/HER | Blocking antibody | Breast cancer |
| Bevacizumab | VEGF | Neutralizing antibody | Colorectal cancer |
VEGF – Bevacizumab: In contrast to the other immunological agents which attach directly to the receptor of the tyrosine kinase, the currently available antibody bevacizumab interrupts the signal cascade by neutralizing the ligand. In a study comprising over 800 patients with metastatic colorectal cancer, the addition of bevacizumab to irinotecan, 5-fluorouracil, and leucovorin as first-line therapy led to a significant increase in survival time[35]. Recent studies concerning the neutralizing antibody bevacizumab, and the small molecule tyrosine kinase inhibitor SU5416, demonstrated that, while unlikely to be effective as monotherapy, incorporation of VEGFR blockade into cytotoxic regimens may increase overall response rates in solid tumors. However, incorporation may also introduce new toxicities, including thromboembolic complications and bleeding[36-38]. Gastrointestinal bleeding, epistaxis and thrombotic events have been seen in 10.3%, 50% and 19.1%, respectively, of the patients receiving bevacizumab alone[39]; these side effects should be taken into consideration when deciding about bevacizumab therapy. There is a phase II trial of bevacizumab, irinotecan, and cisplatin in metastatic or unresectable gastric cancer (National Cancer Institute [NCI] protocol 6447) currently active, of which only the high rate of thromboembolic events in six (25%) of 24 patients (95% CI, 11% to 45%) has recently been reported[40]. However, the incidence of thromboembolic events was not significantly different when compared to a similar irinotecan-based protocol not containing bevacizumab, and there was no difference in the pattern of venous thromboses.
A small molecule inhibitor of VEGFR2 (Vatalanib; PTK787/ZK 222584) has been developed; preliminary data on its use in colorectal cancer exist[41]. With respect to gastric cancer, a Japanese group reported the effects of another VEGFR tyrosine kinase inhibitor (SU6668) in a mouse model of gastric cancer. SU6668 did not directly alter the growth rate of the cancer cells, but inhibited tumor angiogenesis, resulting in the inhibition of tumor dissemination in the peritoneum[42].
Erb B2 / HER-neu – Trastuzumab: When considering how EGFR/erb B-targeted therapeutics function, it is important to mention that, in contrast to the small molecule kinase inhibitors, antibodies targeting EGFR and erb B2 have the inherent ability to recruit immune effector cells such as macrophages and monocytes to the tumor through binding of the antibody constant Fc domain to specific receptors on these cells. In xenograft models at least, this mechanism is relevant with the anti-tumor activity of erb B2-targeted trastuzumab[43]. Whether this mechanism has a role in clinical efficacy in cancer patients remains unproven[44]. Trastuzumab (Herceptin) is a humanized monoclonal antibody, which was approved by the United States FDA in 1998 for the treatment of advanced breast cancer. This was the first approval of a monoclonal antibody for use in solid tumor therapy[45]. Recently, it was investigated whether trastuzumab could affect the growth of HER/neu-overexpressing gastric cancer cells based on the antibody-dependent cell-mediated cytotoxicity (ADCC). It has been demonstrated that these cells could be killed by trastuzumab-mediated ADCC and this correlated with the degree of HER-2/neu expression[46]. However, the trastuzumab-mediated ADCC was significantly impaired when tested in peripheral blood mononuclear cells from patients with advanced disease as compared to those with early disease. Moreover, natural killer cells purified from patients with advanced disease showed less trastuzumab-mediated ADCC in comparison to those from healthy donors. Consequently it has also been postulated that some treatment modalities, such as those involving the use of interleukin 2, could contribute to reverse NK dysfunction, which may be necessary for successful trastuzumab treatment of gastric cancer. Funato et al[47] used MKN-7 and KATO-III gastric cancer cells, which express the erb B2 oncogene, to study the mechanism of resistance to cisplatin. They found that erb B2 expression in gastric cancer is related to cisplatin sensitivity, and that anti-erb B2 antisense oligonucleotides could induce increased sensitivity to this drug. Experimental therapy utilizing a different anti-HER mouse-human chimeric monoclonal antibody named CH401 documented efficacy in an in vivo model of gastric cancer, yet clinical data are lacking[45].
Cetuximab has been approved by the FDA for use in colorectal cancer; cetuximab and other molecules inhibiting the EGFR pathway in colorectal cancer have been recently reviewed[48,49]. One large trial of cetuximab in colorectal cancer comprising 329 patients with irinotecan-refractory metastatic cancer indicated a delayed median time to progression by combining cetuximab with irinotecan[50]. A known adverse characteristic of anti-EGFR therapy is an acne-like skin rash associated with treatment response, which was observed in about three out of four patients[51]. In contrast to gefitinib, the response of cancer cells to cetuximab occurs independent of the mutational status of the EGFR[52,53]. Unfortunately, there are no relevant clinical data available on the use of cetuximab in patients suffering from gastric cancer. As cetuximab is a chimeric antibody which may cause immunological reactions, humanized anti-EGFR antibodies have been developed, one being matuzumab, which currently undergoes phase II trials including studies in patients suffering from gastric cancer[54].
Imatinib: Imatinib is an established and licensed treatment modality in gastrointestinal stroma cell tumors (GIST)[55], but with respect to gastric cancer, only limited data about its use exist. A single study in an animal model of gastric cancer suggested no independent activity of imatinib, but proved an effective chemo- sensitization of antitumor drugs, such as 5-FU and paclitaxel for gastric carcinoma, targeting the PDGF/PDGFR-signalling pathway of tumor cells and stromal cells in disease progression and angiogenesis[25].
The first representative of this drug class to be approved for cancer therapy was gefitinib (Iressa®) in the third line treatment of non-small-cell lung cancer[56]. A subsequent phase III trial (Iressa Survival Evaluation in Lung cancer, ISEL) failed to demonstrate survival advantage for those patients when compared with placebo[57]. Furthermore, in other malignancies such as gastric carcinoma, preliminary data indicate that treatment efficacy with this regimen is limited as well[58]. A phase II trial to investigate the efficacy, tolerability and pharmacokinetics of gefitinib in pre-treated patients with metastatic gastric carcinoma included 75 subjects who were randomised to receive 250 or 500 mg/d gefitinib orally. The authors found that gefitinib monotherapy was generally well tolerated in pretreated patients with gastric metastatic adenocarcinoma, with disease control achieved in 18.3% of cases analyzed. The most common drug-related adverse events were diarrhea, rash and anorexia. The only dose-related adverse events were rash (25.0% at 250 mg/d vs 44.7% at 500 mg/d) and anorexia (8.3% at 250 mg/d vs 15.8% at 500 mg/d). Rojo et al[59]evaluated immunohistochemically the percentage of tumor cells expressing EGFR, pEGFR (the activated phosphorylated form), pMAPK, pAkt (phosphorylated Ser473) and Ki67, before and after treatment with gefinitib. Prior to treatment EGFR expression was found in 62.5% of tumors, whereas pEGFR levels were significantly reduced after the treatment. However, a decreased proliferation was observed only in those tumors with low levels of pAkt, suggesting a role for the PI3k-Akt pathway in gefinitib resistance. Recent studies suggested that clinical response to gefitinib in lung cancer depends on the presence of somatic mutations of the EGF receptor in the tumor which enhance the responsiveness of the receptor to EGF ligand and increase its sensitivity to inhibition by gefitinib[60-62]. In the case of gastric cancer, no such mutations are known. Data on the use of erlotinib (Tarceva®; OSI-744) in gastric cancer is limited to a single study of 70 patients having either gastric cancer (n = 26) or gastroesophageal junction cancer (GEJC) (n = 44). No patient in the gastric cancer cohort presented an objective response, but five patients in the GEJC cohort did so, one being a complete response. An overall response rate was 11%[63]. The best known therapeutics targeting members of the EGFR family which are currently available or under investigation are summarized in Table 2.
| Agent | Type | Target | Status |
| Trastuzumab | Humanized mAb | erbB2 | Approved for breast cancer |
| Pertuzumab | Humanized mAb | erbB2 | Phase II trials |
| Cetuximab | Chimeric mAb | EGFR | Approved for colorectal cancer |
| Matuzumab | Humanized mAb | EGFR | Phase II trials |
| Panitumab | Humanized mAb | EGFR | Trials ongoing |
| Gefitinib | TKI | EGFR | Approved for NSCLC |
| Erlotinib | TKI | EGFR | Approved for NSCLC |
| Lapatinib | TKI | EGFR/erbB2 | Phase III trial / breast cancer |
| AEE788 | TKI | EGFR/ erbB2/ VEGFR | Phase I trials |
| CI-1033 | Irreversible TKI | EGFR/erbB2 | Phase II trials |
| EKB-569 | Irreversible TKI | EGFR/erbB2 | Phase II trials |
| EXEL 7647 / EXEL 0999 | TKI | EGFR/ erbB2/ VEGFR | Phase I trials |
As resistance against single agents may arise and tumor survival may rely on more than one growth factor pathway, several attempts have been made to optimize treatment efficacy, most of them still being in preclinical testing. The following options exist: (1) Combination of inhibitors of the same pathway (e.g. gefitinib and cetuximab) in order to further enhance signal abrogation; (2) To combine inhibitors of different RTK pathways or apply non-selective inhibitors of several pathways in case the cancer cell loses its reliance on one specific pathway; (3) To select cells dependent on growth factor stimulation by use of other cytotoxic drugs or radiation and subsequently eliminate these by an immunological or pharmacological RTKI.
In 2004, two groups described the use of cetuximab combined with gefitinib and erlotinib as enhanced abrogation of the EGFR signal cascade in in vitro as well as in vivo models. Matar et al[64] utilized an EGFR-dependent human tumor xenograft model and found a synergistic effect on cell proliferation and superior inhibition of EGFR-dependent signalling and induction of apoptosis. Even suboptimal doses of gefitinib and cetuximab given together resulted in a complete and permanent regression of large tumors. In the combination-treated tumors, there was a superior inhibition of EGFR, mitogen-activated protein kinase, and Akt phosphorylation, as well as greater inhibition of cell proliferation and vascularization and enhanced apoptosis. Using cDNA arrays, 59 genes could be identified that were coregulated and 45 genes differentially regulated, including genes related to cell proliferation and differentiation, transcription, DNA synthesis and repair, angiogenesis, signalling molecules, cytoskeleton organization, and tumor invasion and metastasis. Huang et al[65] reported similar findings in head and neck tumors and in a model of lung cancer; they observed that gefitinib and erlotinib retained the capacity to inhibit tumor cell growth in case of cetuximab resistance. Again, the combination of antibody and kinase inhibitor resulted in more profound tumor regression and regrowth delay.
Another group applied trastuzumab and an inhibitor of EGFR and erb B2 tyrosine kinases, lapatinib, in erb B2-overexpressing breast cancer cell lines. Only in combination, treatment resulted in a markedly downregulated survivin protein expression and enhanced tumor cell apoptosis thus suggesting a potential improvement in clinical response[66]. But not all findings support the idea of combining inhibitors of the same pathway: in an in vitro model using two human epidermoid cell lines the combination of cetuximab and gefitinib demonstrated antagonistic effects. Administration of either drug alone led to a diminution in EGFR levels, while their combination increased the cellular expression of EGFR. These findings suggest that new and tempting treatment strategies on the EGFR target consisting in a double hit with a monoclonal antibody and a TKI must be considered with caution[67]. Data with regard to gastric cancer are currently lacking.
Cancers possessing complex kinase profiles may respond better to a multimodal therapy tackling several pathways; and such regime may simultaneously reduce the emergence of resistance. Therefore a combination of different agents or a single “unspecific” inhibitor of several pathways may offer advantages over inhibition of a single pathway.
Combinations of different agents: As there is currently no single known inhibitor of all relevant receptor tyrosine kinases, combination of the well evaluated agents offers a straightforward concept of therapy, taking into account that in most cancer therapies different substances are being combined. First data were raised in phase I or II breast cancer trials by combining trastuzumab with small molecule inhibitors of EGFR such as gefitinib or erlotinib indicating that this combination provided a well tolerated targeted therapy with preliminary evidence of antitumor activity[68,69]. As activation of the EGF receptor may induce vessel formation by cellular liberation of VEGF, approaches blocking both pathways possess certain attractiveness. In conditions of limiting VEGF, EGF plays an important role in endothelial cell proliferation, survival, and sprouting of small vessels. Animal data suggest that combined inhibition of EGFR and VEGFR pathways may produce synergistic results and that resistance to EGFR inhibition may be overcome by inhibition of VEGFR tyrosine kinase[70,71]. In an in vivo model, the combination of anti-VEGF-R and anti-EGF-R therapies was effective in inhibiting gastric cancer growth whereas the decrease in tumor growth in mice treated with DC101 (an anti-VEGF-R antibody) or cetuximab alone did not reach statistical significance[72]. As mentioned above, the addition of an inhibitor of the hepatocyte growth factor was able to overcome resistance against gefitinib resulting from the interaction of stromal and cancer cells in an animal model of scirrhous gastric cancer[34].
Inhibitors not selective for a single pathway: Agents targeting multiple RTKs are currently undergoing preclinical testing or phase I studies. These include AEE788 (directed against EGFR, erbB2, VEGFR2; Novartis), BAY 43-9006 (Sorafenib; Raf kinase, VEGFR2, PDGF-R beta; Bayer), SU11248 (PDGF-R, VEGFR, c-kit-R, FLT3-R; SUGEN), and ZD6474 (VEGFR and EGFR, AstraZeneca), all designed to inhibit multiple mechanisms of tumor growth in addition to conventional chemotherapy[73-76]. Similar to the combination of anti-EGFR and anti-VEGFR antibodies mentioned above, McCarthy et al[77] tested the inhibitor of both tyrosine kinases, ZD6474, in an orthotopic model of gastric cancer. The agent led to marked inhibition of tumor growth, tumor cell proliferation, and decrease in microvessel density. The authors concluded that therapies such as ZD6474 that target two distinct aspects of tumor growth, angiogenesis and tumor cell proliferation, warrant further investigation.
“Conventional” chemotherapy and RTKIs: The combination of antibody based therapies with conventional therapy has become a standard procedure in many cancers. For example, in colorectal cancer, cetuximab or bevacizumab is typically combined with irinotecan-based regimes[35,50]. One of the first reports on the use of an RTKI, trastuzumab, in breast cancer documented the inhibition of DNA repair subsequent to DNA damage by cisplatin by an antibody to Her2/Neu. Therapy with this antibody led to a 35%-40% reduction in repair of cisplatin-DNA adducts after cisplatin exposure and, as a result, promoted drug-induced killing in target cells[78]. Another study using human tumor xenografts found that gefitinib caused growth inhibition of tumors and enhancement of the activity of a number of cytotoxic drugs, but neither was dependent on high levels of EGFR expression[79]. In an animal model of pancreatic cancer, inhibiting phosphorylation of EGFR, VEGFR, and PDGFR by appropriate RTKIs in combination with gemcitabine enhanced the efficacy of gemcitabine alone, resulting in inhibition of experimental human pancreatic cancer growth and significant prolongation of survival[80]. Similar results were obtained in a model of estrogen receptor-positive breast cancer in which successful cooperation of the dual erb B1/B2 inhibitor lapatinib with tamoxifen was evidenced[81]. Yet there are pitfalls of combinatorial therapy: the combination of two substances may not always be beneficial; for example, the combination of tamoxifen and trastuzumab in estrogen-receptor positive breast cancer may be less effective than either substance alone possibly due to an increase in erb B2 signalling pathways that occurs when tamoxifen is added to trastuzumab[82]. In case of gefitinib, addition of this RTKI to platin-based chemotherapy in non-small cell lung cancer (INTACT 2 trial) showed no added benefit in survival, TTP, or RR compared with standard chemotherapy alone. This large, placebo-controlled trial confirmed the favorable gefitinib safety profile observed in phase I and II monotherapy trials[83]. Currently only data from animal or in vitro testing exist with respect to gastric cancer; Park et al[84] investigated the effect of gefitinib combined with oxaliplatin, 5-fluorouracil, or paclitaxel in a gastric cancer cell line, SNU-1. This study demonstrated the antitumor activity and a significant cell cycle arresting effect induced by gefitinib in SNU-1 human gastric carcinoma cells, and its synergistic interaction with oxaliplatin and paclitaxel. There are preliminary data on a multicenter phase II study of irinotecan, cisplatin, and bevacizumab in gastric or gastroesophageal adenocarcinoma which indicate an excellent disease control rate of 13/15 cases; in a subset of ten patients with measurable disease who had received at least two cycles of therapy, 5 partial responses, 4 minor responses (15%-29% reduction) and 1 stable disease were observed[85]. Other, less toxic substances have been assessed as well. Thus it has previously been documented that gastric tissue exposed to acetylsalicylic acid (ASA) expresses high levels of EGFR[86,87]. A positive loop regulation between COX and erbB2[88] as well as EGFR[89] has been postulated. Since nonsteroidal anti-inflammatory drugs (NSAIDs) might be a tool of carcinoma prevention in the gastrointestinal tract[90] we investigated the mechanisms of a potential synergism of simultaneous cyclooxygenase (COX)- and EGFR-inhibition. It has previously been described that simultaneous administration of COX- and EGFR-inhibitors exerts tumor preventive effects in nude mice[91]. A combination of these two substances with a protein kinase A (PKA) antisense oligonucleotide was able to eliminate tumors in more than half of the animals treated[92]. Preliminary data from our group indicate that acetylsalicylic acid may modulate the expression and activation of EGFR in gastric cancer cells rendering them more susceptible to gefitinib treatment[93].
Radiation therapy and RTKIs: Radiation causes cell death by induction of cellular injury which may rely on subsequent growth factor/receptor tyrosine kinase activation for repair, the combination of radiation and RTKIs seems to be obvious. Currently, however, only data with respect to experimental therapy, especially in animal models, exist. E.g., She et al[94] examined the effect of addition of gefitinib to radiation therapy in a nude mouse model of different cancers, including lung and breast cancer. Gefitinib significantly enhanced the antitumor action of radiation therapy against the test tumors without significant adverse effects, increasing the therapeutic selectivity of ionizing radiation in these model systems. In a similar model, PTK787/ZK222584, a specific inhibitor of VEGFR tyrosine kinases, was tested. Tumors vascularized by radiation-damaged vessels responded to PTK787/ZK222584 with longer latency and slower growth rate than controls, and a trend toward further increase in necrosis, indicating that irradiated tumor vessels are more susceptible to VEGFR inhibition than unirradiated vessels[95].
Receptor tyrosine kinases participate in several steps of tumor formation including proliferation and metastasis formation. As several of them are upregulated in gastric cancer, they offer potential prime targets for a tailored therapy. Unfortunately, only preliminary data exist on the use of the currently clinically available drugs such as trastuzumab, cetuximab, bevacizumab, gefitinib, erlotinib, and imatinib in the setting of gastric cancer. However, phase II trials are underway to examine the potential of these drugs in adenocarcinoma of the stomach. As RTK inhibitors with a broad range are being developed, the potential usefulness of this drug class is most likely to further increase as preclinical data in models of gastric cancer already indicate their effectiveness. The approach of combining RTKIs with “conventional” means of tumor therapy such as cytostatics and radiation therapy is most likely to find its way into clinical application in near future. RTKIs alone typically only inhibit tumor growth and do not aim at killing the cancer cell, but they might prove to be an effective chemosensitizer of other antitumor drugs as they are able to block the process of cellular restitution following injury caused by radiation or “conventional” cytostatics. Different trial designs may be necessary given that some RTKIs might only work in small subsets of patients (for example, the fate of gefitinib in non-small cell lung cancer) and often improve patients’ condition without leading to an objective response. Genomic or proteomic analysis of aberrations in individual patients may be necessary to decide about a tailored therapy.
S- Editor Pan BR L- Editor Zhu LH E- Editor Liu WF
| 1. | Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14 Suppl 2:ii31-ii36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548-5557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 762] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 3. | Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 804] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 4. | Prenzel N, Zwick E, Leserer M, Ullrich A. Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res. 2000;2:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Müller-Tidow C, Schwäble J, Steffen B, Tidow N, Brandt B, Becker K, Schulze-Bahr E, Halfter H, Vogt U, Metzger R. High-throughput analysis of genome-wide receptor tyrosine kinase expression in human cancers identifies potential novel drug targets. Clin Cancer Res. 2004;10:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Kopp R, Ruge M, Rothbauer E, Cramer C, Kraemling HJ, Wiebeck B, Schildberg FW, Pfeiffer A. Impact of epidermal growth factor (EGF) radioreceptor analysis on long-term survival of gastric cancer patients. Anticancer Res. 2002;22:1161-1167. [PubMed] |
| 8. | García I, Vizoso F, Martín A, Sanz L, Abdel-Lah O, Raigoso P, García-Muñiz JL. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol. 2003;10:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Moon WS, Tarnawski AS, Chai J, Yang JT, Majumdar AP. Reduced expression of epidermal growth factor receptor related protein in gastric cancer. Gut. 2005;54:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ono H, Takeuchi Y, Ukegawa J, Kusayanagi S, Mitamura K, Imawari M. Increased tyrosine kinase activites may lead to the phenotypic differences of gastric cancer cells. Anticancer Res. 2004;24:699-705. [PubMed] |
| 11. | Espinoza LA, Tone LG, Neto JB, Costa RS, Wang QJ, Ballejo G. Enhanced TGFalpha-EGFR expression and P53 gene alterations contributes to gastric tumors aggressiveness. Cancer Lett. 2004;212:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Yonemura Y, Ohoyama S, Kimura H, Kamata T, Matsumoto H, Yamaguchi A, Kosaka T, Miwa K, Miyazaki I. The expression of proliferative-associated nuclear antigen p105 in gastric carcinoma. Cancer. 1991;67:2523-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 525] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 15. | Ougolkov A, Yamashita K, Bilim V, Takahashi Y, Mai M, Minamoto T. Abnormal expression of E-cadherin, beta-catenin, and c-erbB-2 in advanced gastric cancer: its association with liver metastasis. Int J Colorectal Dis. 2003;18:160-166. [PubMed] |
| 16. | Lee JW, Soung YH, Kim SY, Park WS, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH. ERBB2 kinase domain mutation in a gastric cancer metastasis. APMIS. 2005;113:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994-998. [PubMed] |
| 18. | Tamburini A, Tomajer V, Gregorc V, Albarello L, Di Palo S, Arrigoni G, Villa E, StaudacherC . Role of VEGF-A, VEGF-D, COX-2 as prognostic factors in curatively resected gastric cancer. Proc Gastrointestinal Am Soc Clin Oncol Symposium. 2004;Abstract 38. |
| 19. | Pohle T, Shahin M, Domschke W, Konturek JW. Effect of basic fibroblast growth factor on gastric ulcer healing and its own mRNA expression. Aliment Pharmacol Ther. 1999;13:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Nakazawa K, Yashiro M, Hirakawa K. Keratinocyte growth factor produced by gastric fibroblasts specifically stimulates proliferation of cancer cells from scirrhous gastric carcinoma. Cancer Res. 2003;63:8848-8852. [PubMed] |
| 21. | Zhao ZS, Zhou JL, Yao GY, Ru GQ, Ma J, Ruan J. Correlative studies on bFGF mRNA and MMP-9 mRNA expressions with microvascular density, progression, and prognosis of gastric carcinomas. World J Gastroenterol. 2005;11:3227-3233. [PubMed] |
| 22. | Shimizu T, Fujiwara Y, Osawa T, Sakai T, Kubo K, Kubo K, Nishitoba T, Kimura K, Senga T, Murooka H. Orally active anti-proliferation agents: novel diphenylamine derivatives as FGF-R2 autophosphorylation inhibitors. Bioorg Med Chem Lett. 2004;14:875-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Chung CK, Antoniades HN. Expression of c-sis/platelet-derived growth factor B, insulin-like growth factor I, and transforming growth factor alpha messenger RNAs and their respective receptor messenger RNAs in primary human gastric carcinomas: in vivo studies with in situ hybridization and immunocytochemistry. Cancer Res. 1992;52:3453-3459. [PubMed] |
| 24. | Katano M, Nakamura M, Fujimoto K, Miyazaki K, Morisaki T. Prognostic value of platelet-derived growth factor-A (PDGF-A) in gastric carcinoma. Ann Surg. 1998;227:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Kim R, Emi M, Arihiro K, Tanabe K, Uchida Y, Toge T. Chemosensitization by STI571 targeting the platelet-derived growth factor/platelet-derived growth factor receptor-signaling pathway in the tumor progression and angiogenesis of gastric carcinoma. Cancer. 2005;103:1800-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Shawver LK, Schwartz DP, Mann E, Chen H, Tsai J, Chu L, Taylorson L, Longhi M, Meredith S, Germain L. Inhibition of platelet-derived growth factor-mediated signal transduction and tumor growth by N-[4-(trifluoromethyl)-phenyl]5-methylisoxazole-4-carboxamide. Clin Cancer Res. 1997;3:1167-1177. [PubMed] |
| 27. | Ko YJ, Small EJ, Kabbinavar F, Chachoua A, Taneja S, Reese D, DePaoli A, Hannah A, Balk SP, Bubley GJ. A multi-institutional phase ii study of SU101, a platelet-derived growth factor receptor inhibitor, for patients with hormone-refractory prostate cancer. Clin Cancer Res. 2001;7:800-805. [PubMed] |
| 28. | Adamson PC, Blaney SM, Widemann BC, Kitchen B, Murphy RF, Hannah AL, Cropp GF, Patel M, Gillespie AF, Whitcomb PG. Pediatric phase I trial and pharmacokinetic study of the platelet-derived growth factor (PDGF) receptor pathway inhibitor SU101. Cancer Chemother Pharmacol. 2004;53:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 417] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 30. | Soman NR, Correa P, Ruiz BA, Wogan GN. The TPR-MET oncogenic rearrangement is present and expressed in human gastric carcinoma and precursor lesions. Proc Natl Acad Sci U S A. 1991;88:4892-4896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Inoue T, Kataoka H, Goto K, Nagaike K, Igami K, Naka D, Kitamura N, Miyazawa K. Activation of c-Met (hepatocyte growth factor receptor) in human gastric cancer tissue. Cancer Sci. 2004;95:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Heideman DA, van Beusechem VW, Bloemena E, Snijders PJ, Craanen ME, Offerhaus GJ, Derksen PW, de Bruin M, Witlox MA, Molenaar B. Suppression of tumor growth, invasion and angiogenesis of human gastric cancer by adenovirus-mediated expression of NK4. J Gene Med. 2004;6:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Ueda K, Iwahashi M, Matsuura I, Nakamori M, Nakamura M, Ojima T, Naka T, Ishida K, Matsumoto K, Nakamura T. Adenoviral-mediated gene transduction of the hepatocyte growth factor (HGF) antagonist, NK4, suppresses peritoneal metastases of gastric cancer in nude mice. Eur J Cancer. 2004;40:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Namiki Y, Namiki T, Yoshida H, Date M, Yashiro M, Matsumoto K, Nakamura T, Yanagihara K, Tada N, Satoi J. Preclinical study of a "tailor-made" combination of NK4-expressing gene therapy and gefitinib (ZD1839, Iressa) for disseminated peritoneal scirrhous gastric cancer. Int J Cancer. 2006;118:1545-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7734] [Article Influence: 368.3] [Reference Citation Analysis (1)] |
| 36. | Shinkaruk S, Bayle M, Laïn G, Déléris G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2003;3:95-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3:263-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591-595. [PubMed] |
| 39. | Fernando NH, Hurwitz HI. Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Semin Oncol. 2003;30:39-50. [PubMed] |
| 40. | Shah MA, Ilson D, Kelsen DP. Thromboembolic events in gastric cancer: high incidence in patients receiving irinotecan- and bevacizumab-based therapy. J Clin Oncol. 2005;23:2574-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Tyagi P. Vatalanib (PTK787/ZK 222584) in combination with FOLFOX4 versus FOLFOX4 alone as first-line treatment for colorectal cancer: preliminary results from the CONFIRM-1 trial. Clin Colorectal Cancer. 2005;5:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Tokuyama J, Kubota T, Saikawa Y, Yoshida M, Furukawa T, Otani Y, Kumai K, Kitajima M. Tyrosine kinase inhibitor SU6668 inhibits peritoneal dissemination of gastric cancer via suppression of tumor angiogenesis. Anticancer Res. 2005;25:17-22. [PubMed] |
| 43. | Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2060] [Cited by in RCA: 2089] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 44. | Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2397] [Cited by in RCA: 2487] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 45. | Hinoda Y, Sasaki S, Ishida T, Imai K. Monoclonal antibodies as effective therapeutic agents for solid tumors. Cancer Sci. 2004;95:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62:5813-5817. [PubMed] |
| 47. | Funato T, Kozawa K, Fujimaki S, Miura T, Kaku M. Increased sensitivity to cisplatin in gastric cancer by antisense inhibition of the her-2/neu (c-erbB-2) gene. Chemotherapy. 2001;47:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Snyder LC, Astsaturov I, Weiner LM. Overview of monoclonal antibodies and small molecules targeting the epidermal growth factor receptor pathway in colorectal cancer. Clin Colorectal Cancer. 2005;5 Suppl 2:S71-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Chung KY, Saltz LB. Antibody-based therapies for colorectal cancer. Oncologist. 2005;10:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 51. | Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20:1S-13S. [PubMed] |
| 52. | Tsuchihashi Z, Khambata-Ford S, Hanna N, Jänne PA. Responsiveness to cetuximab without mutations in EGFR. N Engl J Med. 2005;353:208-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Mukohara T, Engelman JA, Hanna NH, Yeap BY, Kobayashi S, Lindeman N, Halmos B, Pearlberg J, Tsuchihashi Z, Cantley LC. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J Natl Cancer Inst. 2005;97:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 54. | Kim T. Technology evaluation: Matuzumab, Merck KGaA. Curr Opin Mol Ther. 2004;6:96-103. [PubMed] |
| 55. | Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M, Judson IR. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 56. | Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, Abraham S, Rahman A, Liang C, Lostritto R. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 57. | Thatcher N, Chang A, Parikh P, Pemberton K, Archer V. Results of Phase III placebo-controlled study (ISEL) of gefitinib (IRESSA) plus best supportive care (BSC) in patients with advanced non-small-cell lung cancer (NSCLC) who had received 1 or 2 prior chemotherapy regimens. Proc Am Assoc Cancer Res. 2005;46:Abstract #LB-6. |
| 58. | Doi T, Koizumi W, Siena S, Cascinu S, Ohtsu A, Michael M, Takiuchi H, Swaisland H, Gallagher N, Van Cutsem E. Efficacy, tolerability, and pharmacokinetics of gefitinib ('Iressa', ZD1839) in pretreated patients with metastatic gastric cancer. Proc ASCO. 2004;1036 [Abstract]. |
| 59. | Rojo F, Tabernero J, Van Cutsem E. Pharmacodynamic studies of tumor biopsy specimens from patients with advanced gastric carcinoma undergoing treatment with gefinitib (ZD1839). Proc ASCO. 2003;[Abstract 764]. |
| 60. | Stratton MR, Futreal PA. Cancer: understanding the target. Nature. 2004;430:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7278] [Cited by in RCA: 7497] [Article Influence: 357.0] [Reference Citation Analysis (0)] |
| 62. | Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8739] [Cited by in RCA: 8786] [Article Influence: 418.4] [Reference Citation Analysis (0)] |
| 63. | Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, Hackett CB, Urba SG, Zaner KS, Blanke CD. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922-4927. [PubMed] |
| 64. | Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, Guzmán M, Rodriguez S, Arribas J, Palacios J. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487-6501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 65. | Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355-5362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 66. | Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24:6213-6221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 210] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 67. | Fischel JL, Formento P, Milano G. Epidermal growth factor receptor double targeting by a tyrosine kinase inhibitor (Iressa) and a monoclonal antibody (Cetuximab). Impact on cell growth and molecular factors. Br J Cancer. 2005;92:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Moulder SL, Arteaga CL. A Phase I/II Trial of trastuzumab and gefitinib in patients with Metastatic Breast Cancer that overexpresses HER2/neu (ErbB-2). Clin Breast Cancer. 2003;4:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Britten CD. Targeting ErbB receptor signaling: a pan-ErbB approach to cancer. Mol Cancer Ther. 2004;3:1335-1342. [PubMed] |
| 70. | Raben D, Bianco C, Damiano V, Bianco R, Melisi D, Mignogna C, D'Armiento FP, Cionini L, Bianco AR, Tortora G. Antitumor activity of ZD6126, a novel vascular-targeting agent, is enhanced when combined with ZD1839, an epidermal growth factor receptor tyrosine kinase inhibitor, and potentiates the effects of radiation in a human non-small cell lung cancer xenograft model. Mol Cancer Ther. 2004;3:977-983. [PubMed] |
| 71. | Sini P, Wyder L, Schnell C, O'Reilly T, Littlewood A, Brandt R, Hynes NE, Wood J. The antitumor and antiangiogenic activity of vascular endothelial growth factor receptor inhibition is potentiated by ErbB1 blockade. Clin Cancer Res. 2005;11:4521-4532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, Hicklin DJ, Ellis LM. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Traxler P, Allegrini PR, Brandt R, Brueggen J, Cozens R, Fabbro D, Grosios K, Lane HA, McSheehy P, Mestan J. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931-4941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 74. | Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 662] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 75. | Sun L, Liang C, Shirazian S, Zhou Y, Miller T, Cui J, Fukuda JY, Chu JY, Nematalla A, Wang X. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem. 2003;46:1116-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 423] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 76. | Bates D. ZD-6474. AstraZeneca. Curr Opin Investig Drugs. 2003;4:1468-1472. [PubMed] |
| 77. | McCarty MF, Wey J, Stoeltzing O, Liu W, Fan F, Bucana C, Mansfield PF, Ryan AJ, Ellis LM. ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor with additional activity against epidermal growth factor receptor tyrosine kinase, inhibits orthotopic growth and angiogenesis of gastric cancer. Mol Cancer Ther. 2004;3:1041-1048. [PubMed] |
| 78. | Pietras RJ, Fendly BM, Chazin VR, Pegram MD, Howell SB, Slamon DJ. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene. 1994;9:1829-1838. [PubMed] |
| 79. | Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885-4892. [PubMed] |
| 80. | Yokoi K, Sasaki T, Bucana CD, Fan D, Baker CH, Kitadai Y, Kuwai T, Abbruzzese JL, Fidler IJ. Simultaneous inhibition of EGFR, VEGFR, and platelet-derived growth factor receptor signaling combined with gemcitabine produces therapy of human pancreatic carcinoma and prolongs survival in an orthotopic nude mouse model. Cancer Res. 2005;65:10371-10380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Chu I, Blackwell K, Chen S, Slingerland J. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65:18-25. [PubMed] |
| 82. | Ropero S, Menéndez JA, Vázquez-Martín A, Montero S, Cortés-Funes H, Colomer R. Trastuzumab plus tamoxifen: anti-proliferative and molecular interactions in breast carcinoma. Breast Cancer Res Treat. 2004;86:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22:785-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1284] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 84. | Park JK, Lee SH, Kang JH, Nishio K, Saijo N, Kuh HJ. Synergistic interaction between gefitinib (Iressa, ZD1839) and paclitaxel against human gastric carcinoma cells. Anticancer Drugs. 2004;15:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | Shah MA, Ramanathan RK, Ilson DH, Levnor A, D'Adamo D, O'Reilly E, Tse A, Trocola R, Schwartz L, Capanu M. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201-5206. [PubMed] |
| 86. | Brzozowski T, Konturek PC, Konturek SJ, Stachura J. Gastric adaptation to aspirin and stress enhances gastric mucosal resistance against the damage by strong irritants. Scand J Gastroenterol. 1996;31:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Konturek PC. Physiological, immunohistochemical and molecular aspects of gastric adaptation to stress, aspirin and to H. pylori-derived gastrotoxins. J Physiol Pharmacol. 1997;48:3-42. [PubMed] |
| 88. | Benoit V, Relic B, Leval Xd Xd, Chariot A, Merville MP, Bours V. Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene. 2004;23:1631-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 90. | Jiang XH, Wong BC. Cyclooxygenase-2 inhibition and gastric cancer. Curr Pharm Des. 2003;9:2281-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani CM. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 350] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 92. | Tortora G, Caputo R, Damiano V, Melisi D, Bianco R, Fontanini G, Veneziani BM, De Placido S, Bianco AR, Ciardiello F. Combination of a selective cyclooxygenase-2 inhibitor with epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 and protein kinase A antisense causes cooperative antitumor and antiangiogenic effect. Clin Cancer Res. 2003;9:1566-1572. [PubMed] |
| 93. | Becker JC, Müller-Tidow C, Brandts C, Serve H, Berdel WE, Domschke W, Pohle T. ASA increases the expression of EGFR in gastric carcinoma cells and enhances their response to Iressa, a selective EGFR tyrosine-kinase inhibitor. Gastroenterology. 2003;125:A-171. |
| 94. | She Y, Lee F, Chen J, Haimovitz-Friedman A, Miller VA, Rusch VR, Kris MG, Sirotnak FM. The epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 selectively potentiates radiation response of human tumors in nude mice, with a marked improvement in therapeutic index. Clin Cancer Res. 2003;9:3773-3778. [PubMed] |
| 95. | Zips D, Eicheler W, Geyer P, Hessel F, Dörfler A, Thames HD, Haberey M, Baumann M. Enhanced susceptibility of irradiated tumor vessels to vascular endothelial growth factor receptor tyrosine kinase inhibition. Cancer Res. 2005;65:5374-5379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |