Published online Apr 7, 2006. doi: 10.3748/wjg.v12.i13.2080
Revised: September 25, 2005
Accepted: October 26, 2005
Published online: April 7, 2006
AIM: Recent studies have demonstrated that obesity is the common feature of cryptogenic cirrhosis (CC) and non-alcoholic steatohepatitis. However, there is little information on CC in the region where obesity is not prevalent.
METHODS: The clinical features, and the liver-related morbidity and mortality of CC were analyzed in Japan where the prevalence of obesity is low. Among 652 cirrhotic patients, we identified 29 patients (4.4%) with CC. Of these, 24 CC patients who were followed up for more than 6 months were compared in a case-control study with age-, sex-, and Child-Pugh score-matched controls having cirrhosis of viral etiology.
RESULTS: Obesity (BMI≥25 kg/m2), diabetes mellitus, and hypertriglyceridemia were more frequent, and the visceral fat area was larger in the CC patients than in the controls. The indices of insulin resistance were higher and the serum aminotransferase levels were lower in the CC patients than in the controls. Logistic regression analysis identified the elevated hemoglobin A1c, BMI ≥ 25 kg/m2, and normal aminotransferase levels as independent predictors of CC. Kaplan-Meier analysis demonstrated lower occurrence of hepatocellular carcinoma and higher survival rate in the CC than in the controls in contrast to the similar cumulative probability of liver-related morbidity between those groups.
CONCLUSION: CC more frequently presents with the clinical features suggestive of non-alcoholic steatohepatitis compared with controls even in the region where obesity is not prevalent. The lower occurrence of hepatocellular carcinoma and higher survival rate may indicate an indolent clinical course in CC as compared with viral cirrhosis.
- Citation: Kojima H, Sakurai S, Matsumura M, Umemoto N, Uemura M, Morimoto H, Tamagawa Y, Fukui H. Cryptogenic cirrhosis in the region where obesity is not prevalent. World J Gastroenterol 2006; 12(13): 2080-2085
- URL: https://www.wjgnet.com/1007-9327/full/v12/i13/2080.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i13.2080
Liver cirrhosis is the terminal condition of liver diseases resulting from various etiologies. Despite the recent development of diagnostic tools, no recognizable etiology can be detected in approximately 5% to 31% of cirrhotic patients who are therefore diagnosed as having cryptogenic cirrhosis (CC)[1-3]. Although several explanations such as unknown viral infections, occult alcohol abuse, or burnt out autoimmune hepatitis had been proposed as possible causes of CC, they actually induce CC only in some cases[3,4].
Obesity is an independent risk factor for chronic liver diseases, and liver fibrosis can develop in the obese patients without any known causes of liver diseases [5,6]. Due to the recent increase of the obese population, great attentions have been paid to non-alcoholic steatohepatitis (NASH), which is characterized by obesity and insulin resistance. It has been widely recognized that NASH can progress to liver cirrhosis and hepatocellular carcinoma (HCC)[7]. Several studies have suggested that NASH may be an under-recognized cause of CC, because the prevalence of risk factors for NASH such as obesity and diabetes mellitus is definitely increased in the patients with CC[8-10]. However, these studies have been conducted in the West where obesity is common, and there is little information on CC in the region where the dietary habits are different from those in the West and the prevalence of obesity is low. Because the key component of the association between NASH and CC is obesity[8-10], whether this association is true even in the non-obese population is an important issue.
In Japan where obesity is not prevalent, the clinical characteristics, and the liver-related morbidity and mortality of CC were investigated with a special reference to the association with NASH in this study. The aim was to clarify the clinical features of CC in the region where the prevalence of obesity is low.
We identified 652 cirrhotic patients in whom sufficient data were available to establish the etiology of liver disease from the inpatients’ registry of Nara Medical University since 1990. Liver cirrhosis was diagnosed on the basis of compatible clinical and imaging findings, and/or liver histology. The diagnosis of CC was made only after an exhaustive evaluation of the clinical history and laboratory data, from which no specific etiologies were defined. Patients with histological evidence of other defined causes of liver diseases were also excluded from the diagnosis of CC. Of 29 patients with CC, 24 patients who had been followed up for more than 6 months were classified as the CC group in our case-control study. For patients in CC group, 3 patients with cirrhosis of viral etiology [2 hepatitis C virus (HCV)-related and 1 hepatitis B virus (HBV)-related], age- (within 3 years), sex-, and Child-Pugh score-matched, were identified from the corresponding inpatients’ registry in a consecutive manner. Finally, 24 patients with CC, 48 with HCV-related cirrhosis, and 24 with HBV-related cirrhosis were enrolled in the present case-control study. This study was approved by the Ethical Committee of Nara Medical University and was performed in accordance with the Declaration of Helsinki.
All patients underwent an exhaustive re-evaluation of the following clinical information: past or present evidences of diseases including diabetes mellitus, dyslipidemia, and hypertension; personal history of alcohol intake, intravenous drug use, or blood transfusion, and family history of liver diseases. The height and weight were measured, and the body mass index (BMI) was calculated as weight (kg) divided by squared height (m). The cases with ascites were evaluated after the resolution of ascites. HBV markers (surface antigen/antibody and core antibody) and HCV antibody were negative in all patients with CC. The following laboratory data were also collected at enrollment: total bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase, albumin, γ-globulin, cholinesterase, total cholesterol, triglyceride, prothrombin activity (International normalized ratio: INR), hemoglobin A1c, α1-antitrypsin, iron storage parameters (transferrin saturation and ferritin), copper and ceruloplasmin levels, anti-nuclear antibody, anti-mitochondrial antibody, and anti-liver kidney microsomal antibody. When immune mediated liver disease could not be ruled out, liver histology was evaluated. The fasting blood levels of glucose and insulin were determined, and the homeostasis model assessment parameter of insulin resistance (HOMA-IR) and the quantitative insulin check index (QUICKI) were calculated as the indices of insulin resistance[11,12]. Liver biopsy was performed in 12 of the 24 patients with CC and was used to investigate the histological features suggestive of NASH.
Computed tomograms were recorded at the umbilical level, and the visceral fat areas were measured as described previously [13]. Other tomograms were taken at the level where the liver and spleen were observed in the same slice. The Hounsfield unit of the liver and spleen was determined, and the liver/spleen ratio was calculated as an index of the hepatic fat content [14].
Clinical information including the tumor markers and the imaging studies was monitored every 6 months until data analysis. In cases which lost to follow-up, up-to-date clinical information was investigated by telephone interview and/or contact with the primary care physician. The following outcomes were evaluated; (1) liver-related morbidity including variceal bleeding, ascites, jaundice, and hepatic encephalopathy, (2) occurrence of HCC, (3) mortality and cause of the death. HCC was histologically confirmed or diagnosed by elevation of α-fetoprotein and/or compatible ultrasonographic or computed tomographic findings. Death was considered to be liver-related when it happened in consequence to hepatic failure, variceal bleeding, and/or HCC.
All analyses were performed with StatView 5.0 program (SAS Institute, Cray, NC, USA). Comparisons were made using the two-tailed Student’s t test for quantitative variables and the Chi-square test for qualitative variables. Kaplan-Meier’s method and the log-rank test were used to compare the cumulative probability to liver-related morbidity and mortality. The factors associated with CC were identified using a multivariate logistic regression model. All data are expressed as mean ± SD. P < 0.05 was considered statistically significant.
Among the 652 patients, cirrhosis was attributed to viral etiology in about 80% (HCV-related cirrhosis: 62.9%, HBV-related cirrhosis: 16.6%) and to alcohol in 9.5%. The prevalence of CC was 4.4%. The other cases resulted from various causes such as autoimmune liver diseases, hemochromatosis, Wilson’s disease, Budd-Chiari syndrome, and congestion. The males were predominant in viral and alcoholic cirrhosis, whereas there was no gender difference in CC (male/female ratio; HCV: 255/155, HBV: 82/26, alcohol: 52/10, CC: 15/14).
The follow-up period was not significantly different between groups. Whereas one-third of the patients with viral cirrhosis were transfused, no patient with CC was transfused. BMI was significantly higher in the CC patients than in the controls of viral etiology. AST and ALT levels were lower in the CC patients than in the controls, although AST was higher than ALT in all groups. Cholinesterase, total cholesterol, and triglyceride were higher in the CC patients than in the controls. The fasting levels of blood glucose, hemoglobin A1c, insulin, and the indices of insulin resistance such as HOMA-IR and QUICKI were also higher in the CC patients than in the controls. Iron storage parameters such as transferrin saturation and ferritin were similar in all groups (Table 1). The visceral fat area was larger in the CC patients than in the controls, whereas the liver/spleen ratio was similar between groups. Obesity was more prevalent in CC patients than in controls (BMI ≥ 30 kg/m2: CC 16.7% vs controls 1.4%, P < 0.05) (Table 2). The patients whose visceral fat area was larger than 100 cm2 constituted 40% of the CC as compared with 5.3% of the controls (P < 0.05). Type 2 diabetes mellitus and hypertriglyceridemia were more frequent in the CC patients than in the controls. No patient suffered from type 1 diabetes mellitus. The prevalence of hypertension was similar in all groups.
| Cryptogenic(n=24) | Control; all(n=72) | HCV-related(n=48) | HBV-related(n=24) | |
| Age (yr) | 58.2 ± 10.6 | 58.6 ± 8.8 | 58.7 ± 8.1 | 58.3 ± 10.2 |
| Child-Pugh score | 6.3 ± 1.4 | 6.5 ± 1.2 | 6.5 ± 1.2 | 6.4 ± 1.3 |
| Follow-up (yr) | 5.7 ± 4.2 | 5.9 ± 4.4 | 6.5 ± 4.2 | 4.7 ± 4.5 |
| Transfusion (%) | 0 | 26.8 b | 34.0 b | 12.5 |
| Body mass index (kg/m2) | 25.5 ± 3.2 | 22.4 ± 3.0 b | 22.6 ± 3.3 b | 22.0 ± 2.4 b |
| ICGR15 (%) | 31.5 ± 16.7 | 27.2 ± 12.3 | 28.2 ± 12.1 | 25.0 ± 12.7 |
| Total bilirubin (mmol/L) | 22 ± 15 | 21 ± 10 | 22 ± 10 | 19 ± 10 |
| AST (nkat/L) | 959 ± 572 | 1349 ± 772 a | 1410 ± 573 b | 1229 ± 1064 |
| ALT (nkat/L) | 860 ± 640 | 1229 ± 765 a | 1359 ± 748 b | 972 ± 747 |
| AKP (nkat/L) | 6518 ± 2942 | 6638 ± 2976 | 6695 ± 2577 | 6529 ± 3692 |
| Albumin (g/L) | 40 ± 5 | 38 ± 5 | 38 ± 5 b | 38 ± 6 |
| γ-globulin (g/L) | 17 ± 3 | 18 ± 5 | 19 ± 4 | 15 ± 5 |
| Cholinesterase (U/L) | 242.8 ± 118.2 | 174.5 ± 67.8 b | 162.1 ± 61.7 b | 198.8 ± 73.7 |
| Total cholesterol (mg/dL) | 170.5 ± 43.0 | 156.5 ± 35.1 | 151.2 ± 31.3 b | 166.8 ± 40.2 |
| Triglyceride (mg/dL) | 103.8 ± 44.2 | 83.0 ± 25.5 b | 84.5 ± 26.6 b | 80.1 ± 23.8 b |
| Prothrombin time (INR) | 1.15 ± 0.15 | 1.19 ± 0.14 | 1.21 ± 0.14 | 1.16 ± 0.14 |
| Hemoglobin A1c (%) | 6.2 ± 1.3 | 5.1 ± 0.8 b | 5.3 ± 0.9 a | 4.8 ± 0.7 b |
| Blood glucose (mg/dL) | 123.1 ± 48.2 | 96.4 ± 21.0 b | 96.9 ± 23.0 b | 95.2 ± 16.3 a |
| Insulin (mU/L) | 26.8 ± 15.7 | 14.2 ± 6.4 b | 15.9 ± 6.6 b | 10.8 ± 4.5 b |
| HOMA-R (%) | 8.6 ± 4.8 | 3.6 ± 2.5 b | 4.0 ± 2.9 b | 2.6 ± 1.3 b |
| QUICKI | 0.29 ± 0.02 | 0.33 ± 0.03 b | 0.32 ± 0.02 b | 0.34 ± 0.04 b |
| Transferrin saturation (%) | 36.3 ± 17.5 | 45.2 ± 29.7 | 48.5 ± 31.1 | 40.0 ± 27.8 |
| Ferritin (mg/L) | 160.0 ± 155.3 | 193.5 ± 282.9 | 225.0 ± 318.1 | 99.3 ± 104.8 |
| Visceral fat area (cm2) | 102.0 ± 39.8 | 56.9 ± 35.3 b | 52.1 ± 30.7 a | 64.3 ± 43.9 b |
| Liver/spleen ratio | 1.08 ± 0.05 | 1.08 ± 0.06 | 1.07 ± 0.06 | 1.09 ± 0.06 |
| Cryptogenic(n=24) | Control; all(n=72) | HCV-related(n=48) | HBV-related(n=24) | |
| Body mass index: ≥25kg/m2 | 54.2% | 20.8%b | 29.2%a | 8.3%b |
| ≥30kg/m2 | 16.7% | 1.4%a | 2.1%a | 0%a |
| Visceral fat area: ≥100cm2 | 40.0% | 5.3%b | 3.7%b | 9.1%a |
| Complication | ||||
| Type 2 diabetes mellitus | 54.2% | 26.4%a | 35.4% | 13.3%b |
| Hypertriglyceridemia (≥150mg/dL) | 20.8% | 4.2%a | 2.1%b | 13.3% |
| Hypertension | 25.0% | 15.3% | 18.8% | 13.3% |
Although liver biopsy was performed in 12 of 24 patients with CC, there was no specific finding to define the etiology of liver disease. Ten of 12 biopsies, however, revealed one or more histological components suggestive of NASH such as macrovesicular steatosis, ballooning hepatocyte degeneration, neutrophilic lobular inflammation, and Mallory hyaline. Eight cases had macrovesicular steatosis in less than 30% of the hepatocytes. Ballooning hepatocyte degeneration, neutrophilic lobular inflammation, and Mallory hyaline were present in 7, 2, and 2 cases, respectively. Two cases had no inflammatory activity, 7 cases demonstrated minimal activity, and 3 cases had mild-to-moderate activity. The inflammatory infiltrates consisted predominantly of lymphocytes in fibrous bands.
In the univariate analysis, BMI≥25 kg/m2, visceral fat area ≥ 100cm2, and the coincidence of diabetes mellitus and dyslipidemia, were significantly associated with CC (Table 3). Moreover, normal ALT levels (<667nkat/L), elevated levels of cholinesterase, triglyceride, and hemoglobin A1c ≥ 6.0%, and HOMA-IR ≥ 4% were significantly associated with CC. The multivariate analysis identified the elevated levels of hemoglobin A1c (OR: 7.8, 95% CI: 1.62-37.55, P < 0.05) and BMI (OR: 6.8, 95% CI: 1.41-32.87, P < 0.05), and normal ALT levels (OR: 5.0, 95% CI: 1.12-22.43, P < 0.05) as independent predictors of CC.
| Odds ratio (95% CI) | P value | |
| Univariate | ||
| Body mass index (≥25 kg/m2) | 4.2 (1.51-11.48) | 0.006 |
| Visceral fat area (≥100 cm2) | 12.8 (2.16-75.32) | 0.005 |
| Diabetes mellitus | 3.3 (1.26-8.60) | 0.015 |
| Dyslipidemia | 6.1 (1.33-27.64) | 0.020 |
| Hypertension | 1.8 (0.60-5.70) | 0.285 |
| ALT (<667 nkat/L) | 3.8 (1.42-9.91) | 0.008 |
| Cholinesterase (≥192 U/L) | 3.9 (1.42-10.94) | 0.008 |
| Triglyceride (≥150 mg/dL) | 5.9 (1.29-26.86) | 0.022 |
| Total cholesterol (≥220 mg/dL) | 3.4 (0.77-14.62) | 0.108 |
| HbA1c (≥6%) | 5.5 (1.40-21.75) | 0.015 |
| HOMA-R (≥4%) | 11.6 (2.46-54.45) | 0.002 |
| Ferritin (≥220 ng/dL) | 0.4 (0.11-1.82) | 0.255 |
| Transferrin saturation (≥40%) | 0.4 (0.11-1.82) | 0.158 |
| Multivariate | ||
| HbA1c (≥6.0%) | 7.8 (1.62-37.55) | 0.010 |
| Body mass index (≥25 kg/m2) | 6.8 (1.41-32.87) | 0.017 |
| ALT (<40 U/L) | 5.0 (1.12-22.43) | 0.035 |
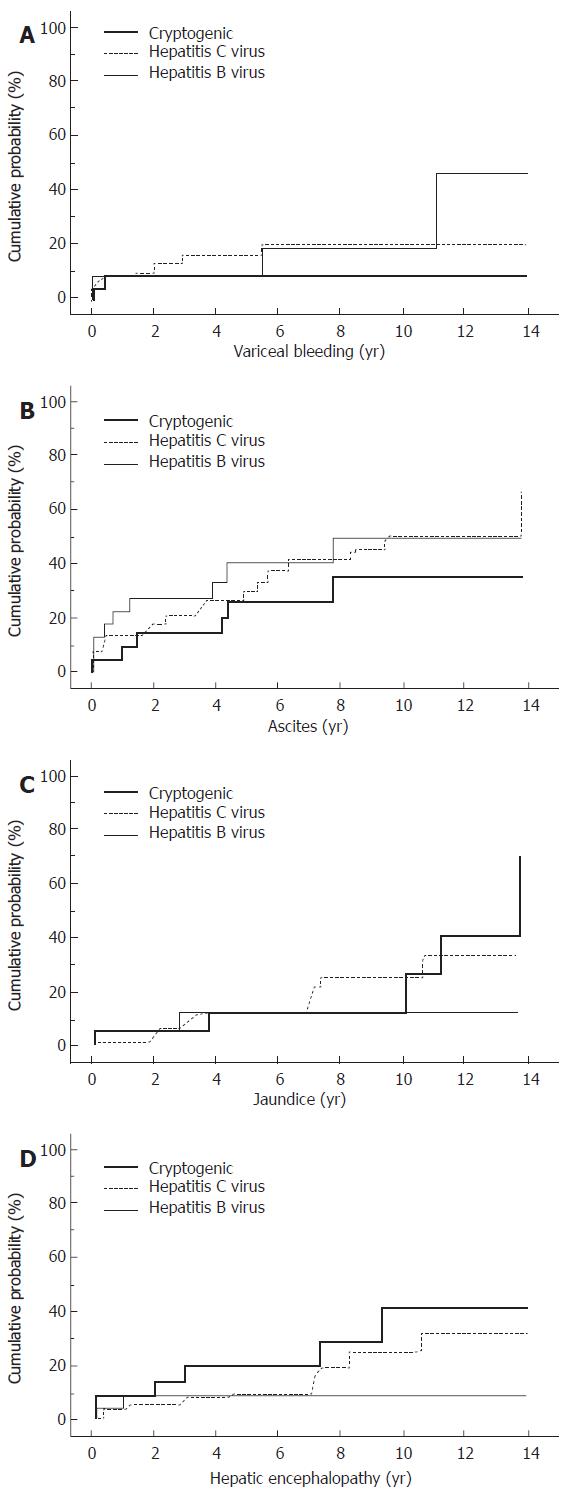
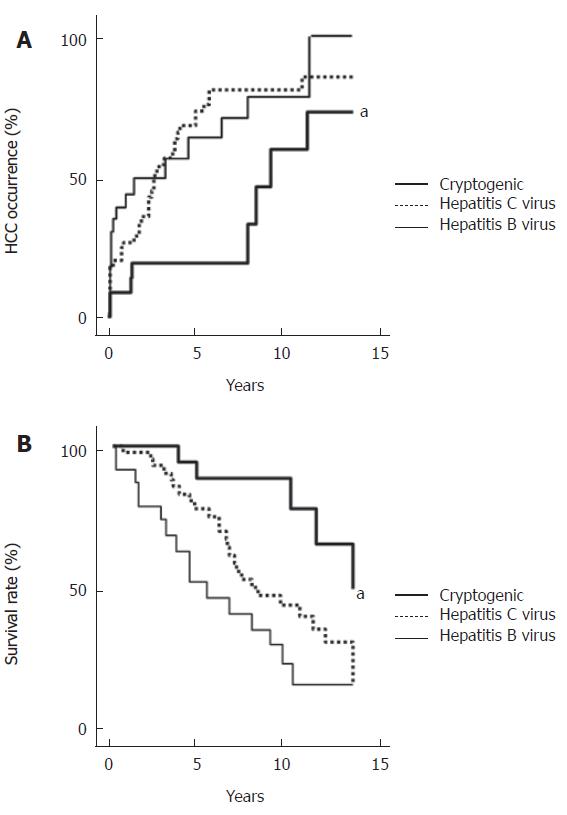
During a mean follow-up of 5.7 years for the CC group (n = 24), 2 patients experienced variceal bleeding, and 6 patients developed ascites (Figure 1). Jaundice and hepatic encephalopathy occurred in 5 patients and 6 patients, respectively. The viral group (n = 72) was followed-up for a mean of 5.9 years, during which variceal bleeding, ascites, jaundice, and hepatic encephalopathy occurred in 12, 27, 20, and 10 cases, respectively. The cumulative probabilities of the liver-related morbidity were not significantly different between the groups. HCC occurred in 9 patients of the CC group in contrast to 53 patients of the controls (HCV: 36 patients, HBV: 17 patients) (Figure 2A). Although the cumulative probability of HCC occurrence was lower in the CC group than in the controls of viral etiology (P < 0.01, CC vs HCV and CC vs HBV), HCC occurrence in the CC group rapidly increased 8 years after the observation. Of 24 patients with CC, 3 died of liver failure and 2 of HCC (Figure 2B). No patient was associated with non-liver-related death. In the controls, 39 patients (24 HCV-related and 15 HBV-related) were associated with liver-related death (liver failure in 10 cases and HCC in 29 cases). The cumulative probability of survival censoring non-liver related deaths were higher in the CC group than in the controls (P < 0.05, CC vs HCV, P < 0.01, CC vs HBV).
Recent studies in the West have proposed that NASH may be an under-recognized cause of CC[8-10]. Although obesity is the key component of the association between CC and NASH, whether this association can be true even in the region with the low prevalence of obesity is unknown. The World Health Organization defined obesity as BMI ≥ 30[15], but in Japan, the prevalence of the population with such BMI is no more than 2%-3% in contrast to the 20%-30% in the West[16-18]. Moreover, the medical examination in 2002 (n = 6360) in the local district where this study was performed showed that the prevalence of BMI ≥ 30 was 2.5% (data not published). We, therefore, investigated the clinical features of CC focusing on the association with NASH in Japan. This study demonstrated that in Japan, obesity, diabetes mellitus, and hypertriglyceridemia were more frequent, and insulin resistance, which is closely associated with the pathogenesis of NASH was greater in the CC group than in the controls of viral etiology. These findings are similar to those in the West, indicating that CC has the similar clinical features to NASH regardless of the prevalence of obesity.
The Japanese people as a race are known to suffer from obesity-related disorders even with a mild excess of adiposity[16,19-21]. The definition of obesity is proposed as BMI ≥ 25 in Japan, because the obesity-related disorders increase with a BMI ≥ 25[16,19]. This study demonstrated that the prevalence of BMI ≥ 30 in the CC group was 16.7% in Japan in contrast to 47% in the West. The prevalence of BMI ≥ 25 in the CC group was 54.2%, which is similar to that of BMI ≥ 30 in the West[9]. Moreover, our patients with CC, despite of the low prevalence of obesity, were accompanied with diabetes mellitus in 54.2% and hypertriglyceridemia in 20.8%, which is also similar to the reports in the West[8,9]. Although the BMI in viral cirrhosis was also lower in Japan than in the West (22.4 ± 3.0 vs 25.1 ± 4.2), the prevalence of diabetes mellitus was similar in these regions (26.4% vs 32.0%)[9]. Considering that the NASH patients in Japan are not as obese as those in the West [21], Japanese people, even with mild obesity, may suffer from NASH which may progress to CC.
Many researchers have shown that excess visceral fat is more closely related to the risk of health problems than the BMI itself[22-23]. The contribution of visceral fat area is greater in Japanese in whom the degree of whole fat accumulation is not as severe as in the West. Our present study demonstrated an increased visceral fat deposit in the CC group as compared with viral cirrhosis group. Because the increased visceral fat deposit plays a role in the pathogenesis of NASH via a production of various adipocytokines from the visceral fat tissue[23,24], the larger visceral fat area in CC further supports the association between CC and NASH. On the other hand, the hepatic fat deposit reflected by the liver/spleen ratio was similar in CC and viral cirrhosis groups. The hepatic fat deposit may decrease in the cirrhotic stage because the sinusoidal capillarization impairs the movement of gut-derived lipoproteins into the hepatocytes and the porto-systemic shunt diverts blood-borne lipids away from the liver. In fact, loss of the hepatic fat deposit has been observed in serial biopsies of NASH patients with progression to cirrhosis[7]. Because the excessive steatosis of the hepatocytes induces oxidative stress leading to the death of hepatocytes, loss of the hepatic fat deposit may be associated with lower ALT levels in CC.
This study demonstrated the lower occurrence of HCC and the higher survival rate in the CC than in viral cirrhosis, indicating that CC may take an indolent clinical course. There is a wide variation in the carcinogenic risk of CC among previously studies. Caldwell et al[8] showed that HCC developed in one of 71 patients with CC (1.4%). Another group reported higher prevalence of HCC in the CC group than in HCV-related cirrhosis[10]. The reason for these controversial data is unclear, but it may be attributed to the difference of the observation period. Bugianesi
et al[25] suggested a later onset of HCC in the CC, because the patients with the CC-associated HCC were older as compared with the other cirrhosis-associated HCCs. Interestingly, this study demonstrated that although the cumulative probability of HCC occurrence was lower in the CC than in the viral cirrhosis, HCC occurrence in the CC rapidly increased 8 years after the observation, indicating the later increase of HCC in CC. The later occurrence of HCC in CC can not be attributed to age and sex difference or degree of liver damage, because of a case-control study with age-, sex-, and Child-Pugh score matched viral cirrhosis. Therefore, HCC occurrence may be a late complication of CC.
In conclusion, the features suggestive of NASH were more frequently observed in the CC patients than in the controls of viral etiology even in Japan where obesity is not prevalent. It indicates that NASH may be a possible cause of CC regardless of the prevalence of obesity. Moreover, HCC developed less frequently and its prognosis was less severe in the CC group, indicating that CC may take an indolent clinical course. Further larger studies are necessary to understand the clinical features of CC.
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
| 1. | Kodali VP, Gordon SC, Silverman AL, McCray DG. Cryptogenic liver disease in the United States: further evidence for non-A, non-B, and non-C hepatitis. Am J Gastroenterol. 1994;89:1836-1839. [PubMed] |
| 2. | Greeve M, Ferrell L, Kim M, Combs C, Roberts J, Ascher N, Wright TL. Cirrhosis of undefined pathogenesis: absence of evidence for unknown viruses or autoimmune processes. Hepatology. 1993;17:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, Khettry U. Cryptogenic cirrhosis: clinicopathologic findings at and after liver transplantation. Hum Pathol. 2002;33:1098-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Uchida T, Shimojima M, Gotoh K, Shikata T, Tanaka E, Kiyosawa K. "Silent" hepatitis B virus mutants are responsible for non-A, non-B, non-C, non-D, non-E hepatitis. Microbiol Immunol. 1994;38:281-285. [PubMed] |
| 5. | Del Gaudio A, Boschi L, Del Gaudio GA, Mastrangelo L, Munari D. Liver damage in obese patients. Obes Surg. 2002;12:802-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 646] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820-826. [PubMed] |
| 8. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 751] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 9. | Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 335] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, Giral P, Grimaldi A, Opolon P, Poynard T. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24508] [Article Influence: 612.7] [Reference Citation Analysis (0)] |
| 12. | Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 586] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 13. | Ogawa Y, Murata Y, Nishioka A, Inomata T, Yoshida S. Tamoxifen-induced fatty liver in patients with breast cancer. Lancet. 1998;351:725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S. A novel technique for the determination of body fat by computed tomography. Int J Obes. 1983;7:437-445. [PubMed] |
| 15. | Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452. [PubMed] |
| 16. | Yoshiike N, Kaneda F, Takimoto H. Epidemiology of obesity and public health strategies for its control in Japan. Asia Pac J Clin Nutr. 2002;11 Suppl 8:S727-S731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4389] [Cited by in RCA: 4037] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 18. | Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1800] [Cited by in RCA: 1659] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 19. | New criteria for 'obesity disease' in Japan. Circ J. 2002;66:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1422] [Article Influence: 61.8] [Reference Citation Analysis (1)] |
| 20. | Nakamura T, Adachi H, Hirai Y, Satoh A, Ohuchida M, Imaizumi T. Association of plasminogen activator inhibitor-1 with insulin resistance in Japan where obesity is rare. Metabolism. 2003;52:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Nozaki Y, Saibara T, Nemoto Y, Ono M, Akisawa N, Iwasaki S, Hayashi Y, Hiroi M, Enzan H, Onishi S. Polymorphisms of interleukin-1 beta and beta 3-adrenergic receptor in Japanese patients with nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2004;28:106S-110S. [PubMed] |
| 22. | Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 840] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 23. | Yatagai T, Nagasaka S, Taniguchi A, Fukushima M, Nakamura T, Kuroe A, Nakai Y, Ishibashi S. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 235] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin. Hepatology. 2004;40:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 692] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 25. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1018] [Article Influence: 44.3] [Reference Citation Analysis (0)] |