Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1798
Revised: September 25, 2005
Accepted: October 26, 2005
Published online: March 21, 2006
We herein report the case of an idiopathic liver cystic mass which aggressively infiltrated the thoraco-abdominal wall. A 74-year-old woman who had a huge cystic lesion in her right hepatic lobe was transferred to our hospital for further examinations. Imaging studies revealed a simple liver cyst, and the cytological findings of intracystic fluid were negative. She was followed up periodically by computed tomography (CT) scans. Seven years later, she complained of a prominence and dull pain in her right thoraco-abdominal region. CT revealed an enlargement of the cystic lesion and infiltration into the intercostal subcutaneous tissue. We suspected the development of a malignancy inside the liver cyst such as cystadenocarcinoma, and she therefore underwent surgery. A tumor extirpation was performed, including the chest wall, from the 7th to the 10th rib, as well as a right hepatic lobectomy. Pathologically, the lesion consisted of severe inflammatory change with epithelioid cell granuloma and bone destruction without any malignant neoplasm. No specific pathogens were evident based on further histological and molecular examinations. Therefore the lesion was diagnosed to be a destructive granuloma associated with a long-standing hepatic cyst. Since undergoing surgery, the patient has been doing well without any signs of recurrence.
- Citation: Kawashita Y, Kamohara Y, Furui J, Fujita F, Miyamoto S, Takatsuki M, Abe K, Hayashi T, Ohno Y, Kanematsu T. Destructive granuloma derived from a liver cyst: A case report. World J Gastroenterol 2006; 12(11): 1798-1801
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1798.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1798
A liver cyst is a common lesion that tends to be mostly asymptomatic, however, once it is associated with other events such as bleeding, infection and rupture, it often becomes symptomatic and treatment is thus usually needed. Granulomas can be seen in any part of the body and they reflect a host inflammatory responses elicited by a wide variety of stimuli[1,2], however, it is uncommon that a granulomatous reaction develops in a liver cyst[3].
We experienced the case of highly destructive, non-malignant, granulomatous lesion that originated from a long-standing hepatic cyst that infiltrated the thoraco-abdominal wall mimicking hepatic cystadenocarcinoma. To the best of our knowledge, this is the first known occurrence of a case of a hepatic granuloma that developed secondary to a liver cyst with a highly invasive capacity.
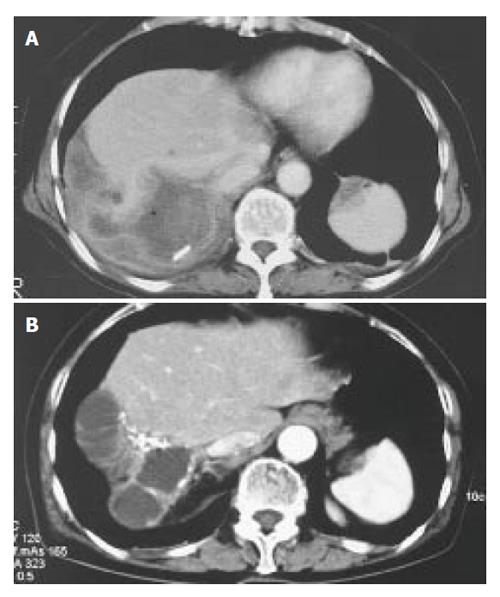
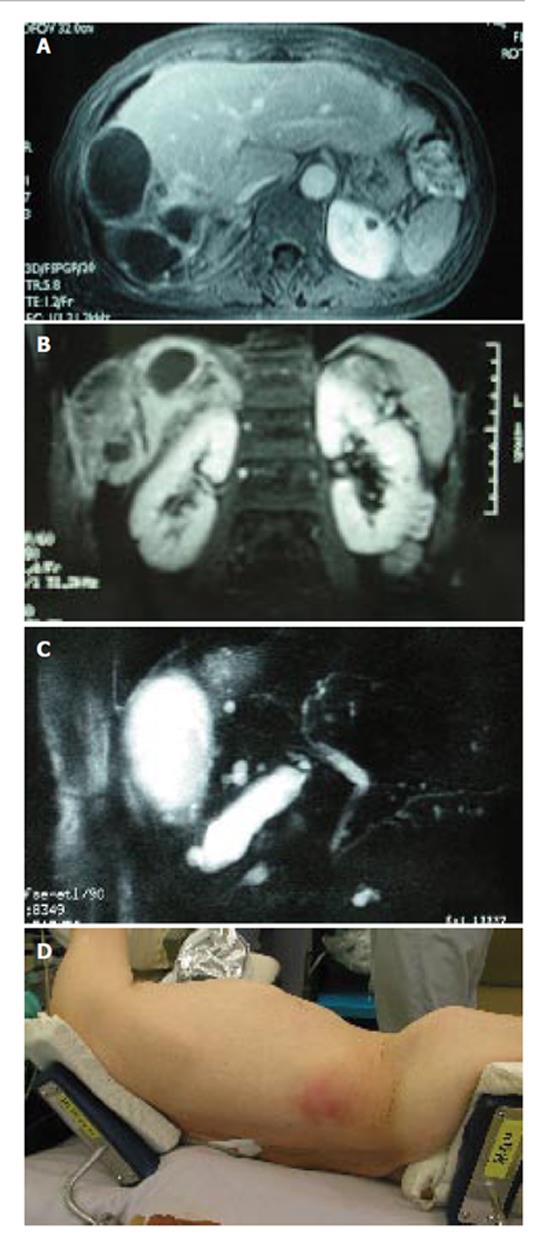
A 73-year-old Japanese woman presented at our hospital with right. hypochondralgia on September 1997. Computed tomography (CT) showed a huge liver cyst measuring 10cm in the right lobe of the liver without any solid component (Figure 1A). Subsequently, 2.4L of serous fluid were percutaneously drained, without any sclerosing agent administered into the cyst. A cytological examination of the aspirated fluid was negative for neoplastic cells. Since her symptom had subsided after the fluid drainage, she was carefully followed-up without any further treatment. On May 2003, she was again referred to us with abdominal distention and pain in the right thoraco-abdominal region. The results of laboratory tests showed slight elevations in the C-reactive protein (CRP) level (0.5mg/dL, normal range < 0.17). Serological tests for hepatitis B and C virus were negative. The serum levels of carcinoembryonic antigen, carbohydrate antigen 19-9 and α-fetoprotein were all within the normal ranges. Other tests including her liver function were within the normal limits. A CT scan showed a cyst in the right. lobe of the liver similar to the one observed 6 years previously. In addition, a calcified lesion was also found around the cyst (Figure 1B). An axial and coronal view of an MRI showed a multilocular cyst with a thick wall and solid component extending into the subcutaneous tissue (Figure 2A and B). MR cholangio-pancreatography demonstrated no dilatation in the biliary system, thus indicating that there was most likely no communication with the liver cyst (Figure 2C). These findings closely resembled hepatic cystadenocarcinoma.
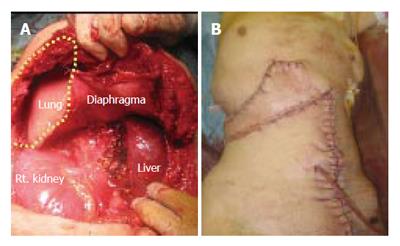
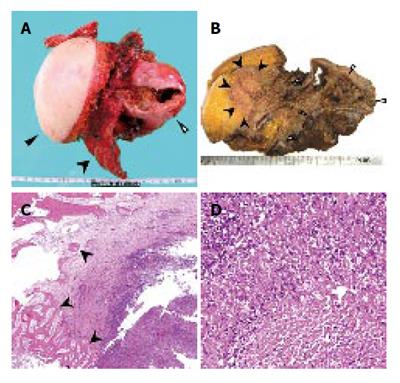
After admission, the cystic tumor gradually increased in size and finally her skin above the invasion site became reddish and swollen (Figure 2D), thus suggesting malignant tumor invasion. After obtaining the patient’s informed consent, we performed an en bloc resection of the hepatic right lobe including the whole cyst, along with the diaphragm, rib bone, and skin. After completing the resection, the defect of the diaphragm was covered with the great omentum, and the thoraco-abdominal wall defect was reconstructed using a musculo-cutaneous flap including the anterior sheath and the left rectus abdominis muscle which both receive the blood supply from the superior abdominal artery (Figure 3A and B). Macroscopically, the cystic tumor directly invaded the intercostal space through the diaphragm (Figures 4A and 4B). No microorganism was found in the cyst fluid or the resected materials according to the results of microbilological culture examinations. A pathological examination revealed that the bone had been destroyed by the invasion of the granuloma, which was composed of epithelioid cells with necrotic areas (Figure 4C). No malignancy or microorganism was detected in any tissue samples. Because the histology was similar to tuberculosis, Ziehl-Neelsen staining and DNA-PCR using three different primers for a tubercle bacillus were performed with negative results. Hepatic hydatid disease was excluded by the fact that she had no history of living or traveling to the epidemic area such as Hokkaido prefecture in Japan and her blood samples did not show the presence of the antibodies against ehinococcus. From these findings, the cystic tumor was therefore histologically diagnosed to be a hepatic epithelioid granuloma. (Figure 4D)
The patient had an uneventful postoperative course and was discharged on postoperative day 27. She remains asymptomatic until 24 mo postoperatively, without any signs of recurrence.
There has been a great progress in the diagnostic modalities for hepatic lesions including ultrasonography, CT, magnetic resonance imaging (MRI) and angiography[4]. Cystic lesions of the liver, which occupy a large part of hepatic lesions and are seen in up to 5% of the population, can be classified as developmental, neoplastic, inflammatory, or miscellaneous lesions. If a solid component develops inside the cyst, the possibility of a malignant transformation should be considered[5]. However, even routine imaging procedures such as CT or MRI generally fail to differentiate hepatic granulomas from other neoplasms[2].
Aspiration cytology can help in making a preoperative diagnosis, however, it is sometimes difficult to accurately hit the target tumor under US guidance, and this procedure also carries a risk of potentially causing needle-track and peritoneal seeding if the lesion is a malignant tumor[6,7].
A wide variety of underlying conditions such as sarcoidosis[8], malignant lymphoma[9], tuberculosis[2], and HCV infection[10,11] can cause hepatic granulomas, with resulting prognostic and therapeutic implications. However, the histological features of such granulomas are not distinctive, while a specific etiological agent often cannot be identified despite serological, immunological microbiological, and radiological investigations, thus often resulting in a diagnosis of “idiopathic” hepatic granulomas. Although the exact etiology and pathogenesis of this aggressively infiltrating granuloma seen in the present case remains unclear, we speculated that an unidentified organism infection possibly occurred via two different routes: (1) the endogenous route, via the blood stream; (2) the exogenous route: a reverse infection through a drainage catheter when aspiration had previously been performed.
A spontaneous regression occurs in some cases of hepatic granulomas, therefore, patients can be treated by simple observation or conservative therapy with anti-inflammatory drugs including antibiotics or steroids in most cases. In the present case, the fact that destroyed and necrotic tissue caused inflammatory reactions suggested that empirical antibiotic therapy would be ineffective, to make matters worse, such conservative treatment could have possibly induced the development of such antibiotic-resistant bacteria as Methicillin-Resistant Staphylococcus Aureus (MRSA). In addition, steroid treatment is generally effective for sarcoidosis, however, it risks exacerbating tuberculosis and an earlier paper described a patient who died from miliary TB after the administration of empirical steroids [12].
Great improvements in operative techniques, perio-perative patient management, and patient selection criteria have not made a hepatic resection one of the standards and effective methods for liver cysts[13]. As a result, a hepatectomy is recommended for cystic lesions when a tumor of the liver with a potential malignancy cannot be ruled out[14,15].
In the present case, the clinical course in which a solid component developed during the follow-up of a liver cyst, while infiltrating into the subcutaneous tissue with the destruction of the ribs was highly suggestive of the development of a malignancy, such as cystadenocarcinoma. The pathological diagnosis turned out to be a cancer-free lesion, namely, benign granuloma. Despite the highly invasive capacity of this lesion, the good clinical outcome in this case supports the suitability of a hepatic resection with the total removal of the affected areas as the treatment of choice.
S- Editor Wang J L- Editor Zhang JZ E- Editor Ma WH
| 1. | Conway KP, Denholm RB, Harrison GA, O'Riordan B. Granulomatous peritonitis in hydatid disease. J Infect. 2003;46:65-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Gaya DR, Thorburn D, Oien KA, Morris AJ, Stanley AJ. Hepatic granulomas: a 10 year single centre experience. J Clin Pathol. 2003;56:850-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Gundogdu ZH, Senocak ME, Caglar M, Buyukpamukcu N. Isolated hepatic granuloma mimicking congenital simple cyst of the liver possibly caused by tuberculosis. J Pediatr Surg. 1992;27:1553-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Ferrucci JT. Liver tumor imaging. Cancer. 1991;67:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Hsieh CB, Chen CJ, Yu JC, Chang TM, Gao HW, Liu YC. Primary squamous cell carcinoma of the liver arising from a complex liver cyst: report of a case. Surg Today. 2005;35:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Yoshida T, Nishimori I, Kumon M, Kohsaki T, Taniuchi K, Ohtsuki Y, Onishi S. Inflammatory pseudotumor of the liver: report of a case diagnosed by needle biopsy. Hepatol Res. 2003;27:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary. Liver Transpl. 2000;6:67-72. [PubMed] |
| 8. | Valla DC, Benhamou JP. Hepatic granulomas and hepatic sarcoidosis. Clin Liver Dis. 2000;4:269-285, ix-x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Kim H, Dorfman RF, Rosenberg SA. Pathology of malignant lymphomas in the liver: application in staging. Prog Liver Dis. 1976;5:683-698. [PubMed] |
| 10. | Ozaras R, Tahan V, Mert A, Uraz S, Kanat M, Tabak F, Avsar E, Ozbay G, Celikel CA, Tozun N. The prevalence of hepatic granulomas in chronic hepatitis C. J Clin Gastroenterol. 2004;38:449-452. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Yamamoto S, Iguchi Y, Ohomoto K, Mitsui Y, Shimabara M, Mikami Y. Epitheloid granuloma formation in type C chronic hepatitis: report of two cases. Hepatogastroenterology. 1995;42:291-293. [PubMed] |
| 12. | Millar JW, Horne NW. Tuberculosis in immunosuppressed patients. Lancet. 1979;1:1176-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 157] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Shimada M, Takenaka K, Gion T, Fujiwara Y, Taguchi K, Kajiyama K, Shirabe K, Sugimachi K. Treatment strategy for patients with cystic lesions mimicking a liver tumor: a recent 10-year surgical experience in Japan. Arch Surg. 1998;133:643-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (74)] |
| 15. | Hofstetter C, Segovia E, Vara-Thorbeck R. Treatment of uncomplicated hydatid cyst of the liver by closed marsupialization and fibrin glue obliteration. World J Surg. 2004;28:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |