Published online Mar 21, 2006. doi: 10.3748/wjg.v12.i11.1766
Revised: October 17, 2005
Accepted: November 10, 2005
Published online: March 21, 2006
AIM: To examine the effects of cyclin D1 antisense oligodexoyneucleotides (ASODN) on growth and chemosensitivity of gastric carcinoma cell lines SGC7901 and its mechanism.
METHODS: Phosphorothioate modified cyclin D1 ASODN was encapsulated by LipofectAMINE2000 (LF2000) and transfected into cells, the dose-effect curves and growth curves were observed. 5-FU, MTX, CDDP of different concentrations were given after transfecting cells with cyclin D1 ASODN for 24 h,the dose-effect responses were observed and IC50s were calculated. The mRNA expression of cyclin D1, thymidylate synthase (TS), thymidine phosphorylase (TP) and dihydrofolate reductase (DHFR) was detected by reverse transcription-PCR (RT-PCR) at 24 h and 48 h after transfection.
RESULTS: Dose-dependent inhibitory effect was caused by cyclin D1 ASODN in SGC7901 cells. Transfecting gastric carcinoma cells with 0.2 µmol/L cyclin D1 ASODN for 24 h could inhibit growth significantly and reduce expression of cyclin D1 mRNA. Cyclin D1 ASODN could increase the chemosensitivity to 5-FU, MTX, CDDP in cells, The IC50s of different chemotherapeutic agents in ASODN plus chemotherapy groups were significantly lower than those in controls. Transfection with cyclin D1 ASODN leaded to an increase in TS and DHFR mRNA and a decrease in TP mRNA as determined by RT-PCR at 24 h, the alterations were more significant at 48 h.
CONCLUSIONS: Cyclin D1 ASODN can decrease mRNA expression of cyclin D1,inhibit growth and enhance the chemosensitivity by changing the expression of enzymes related to metabolism of chemotherapeutic agents in SGC7901 gastric carcinoma cells.
- Citation: Shuai XM, Han GX, Wang GB, Chen JH. Cyclin D1 antisense oligodexoyneucleotides inhibits growth and enhances chemosensitivity in gastric carcinoma cells. World J Gastroenterol 2006; 12(11): 1766-1769
- URL: https://www.wjgnet.com/1007-9327/full/v12/i11/1766.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i11.1766
Cyclin D1 involves in the regulation of G1 phrase in cell cycle, with three subtypes (D1, D2, D3), cyclin D1 located in 11q13, cDNA is 4.3 Kb, is identified as the proto-oncogene and overexpressed in breast, esophageal, and hepatic carcinoma. Cyclin D1 is an important cell cycle regulatory protein that is expressed in the early G1 phase. This protein, in association with the cyclin-dependent kinases CDK4 and CDK6, mediates the phosphorylation of pRb [1]. In this study, we transfected gastric carcinoma cells with cyclin D1 antisense oliogodeoxyneucletides,to observe the effects of it on growth and chemosensitivity to 5-fluorouracil (5-FU),methotrexate (MTX) and cisplatin (CDDP), furthermore, we examined the expression of enzymes related to metabolism of chemotherapeutic agents, including TS, TP and DHFR, to find out the underlying mechanism.
Human moderately differentiated gastric adenocarcinoma cell line SGC7901 was kindly gifted by the Prof. Daiming Fan (the Fourth Military Medical University, Xi’an, China). Cells were cultured in DMEM medium (GIBCO) with 10% newborn calf serum (GIBCO) at 37 °C in 50mL/L CO2. Cyclin D1 antisense oliogodeoxyneucletide 5’-GGA GCT GGT GTT CCA TGG-3’ (ASODN) was complementary to the translation start site of the cyclin D1 cDNA and sense oligomers 5’-CCA TGG AAC ACC AGC TCC-3’ (SODN) was used as control[2], the two sequences were synthesized and phosphorothioate modified by Shanghai Shengong Biotechnology Corp. The lyophilized ODNs was diluted by sterile water and filtered, storaged in -20 °C. Lipofectamine2000 (LF2000, Invitrogen)-ODN complexes were prepared as described in the instruction. 5-FU, MTX and CDDP were purchased from SIGMA.
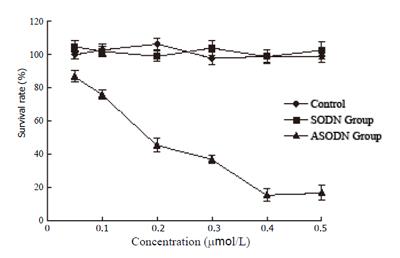
Cells were plated in 96-well plates, SODN and ASODN were mixed with LF2000 respectively, diluted to a increasing concentration (from 0.05 to 0.5 µmol/L) and added to plates, after transfection for 24 h, the media were replaced with DMEM contain serum and cells were continue cultured for 72 h. The viability was determined by MTT assay and survival rates were calculated. The optimal concentration, able to inhibit cell growth of at least 50% vs control, was selected for further experiments.
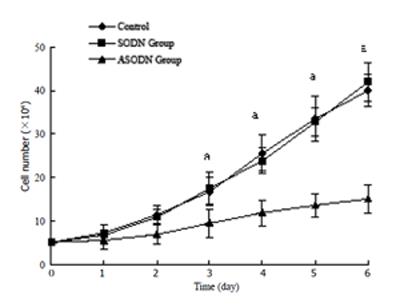
Cells were plated in 12-well plates, treated with LF2000-SODN or ASODN complexes at concentration of 0.2 µmol/L, after transfection for 24 h, replaced with media with serum and cultured for 6 d, cell numbers were counted everyday and the growth curves were drawn.
Cells were plated in 96-well plates and divided into three groups: (1) chemotherapy group, (2) SODN + chemotherapy group, (3) ASODN + chemotherapy group. After transfection with 0.2 µmol/L LF2000-ASODN or LF2000-SODN complexes for 24 h, cells were incubated with medium contain chemotherapeutic agents (5-FU, MTX or CDDP) for 72 h. Cell variability was examined by MTT assay, and dose-effect curves were drawn. The IC50s were calculated by software Prism3 ( Graphpad, USA).
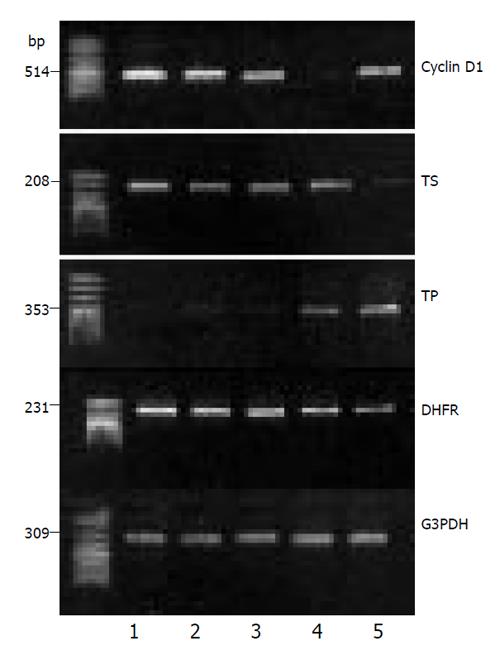
Cells were plated in 6-well plates, transfected and cultured as described, the cells were harvested at 24h and 48h after transfection. Total RNA was isolated by TRIZOL Reagent (GIBCO). Reverse transcription was carried out on 1 µg of total RNA, 20 µL reaction system contained oligo(dT) 0.1µg, 2 mmol/L dNTPs 4 µL, Rnasin 0.1µg, MMLV 200U, incubated at 42 °C for 1h and enzymes were inactivated at 95 °C for 5min. 2.5µL RT products were used for PCR reaction, the dNTPs, sense and antisense primers (100 pmol), Taq DNA polymerase were added into reaction system of 40 µL. G3PDH was used as the control, primers and PCR condition as follow(Table 1)
| Primers | Length of amplification | |
| Cyclin D1 | 5’-GGA TGC TGG AGG TCT GCG AGG AAC-3’ | 514 |
| 5’-GAG AGG AAG CGT GTG AGG CGG TAG-3’ | (332--845) | |
| TS | 5’-CAC ACT TTG GGA GAT GCA CAT ATT T -3’ | 208 |
| 5’-CTT TGA AAG CAC CCT AAA CAG CCA T-3’ | (853--1060) | |
| TP | 5’-ACA AGG TCA GCC TGG TCC TC-3’ | 353 |
| 5’-TCC GAA CTT AAC GTC CAC CAC -3’ | (491--834) | |
| DHFR | 5’-TCC ATT CCT GAG AAG AAT CGA CCT T-3’ | 231 |
| 5’-CAC AAA TAG TTT AAG ATG GCC TGG G-3’ | (657--887) | |
| G3PDH | 5’-TCC CTC AAG ATT GTC AGC AA-3’ | 309 |
| 5’-AGA TCC ACA ACG GAT ACA TT-3’ |
PCR conditions After denaturation for 4 min at 94 °C, thirty-five cycles were accomplished as follow: Cyclin D1, 94 °C, 60 s → 65 °C, 1.5 min→ 72 °C, 1.5 min; TS, 94 °C, 30 s → 55 °C, 30 s → 72 °C, 30 s; TP, 94 °C, 3 0s→ 60 °C, 30 s → 72 °C, 30 s; DHFR, 94 °C, 30 s → 60 °C, 30 s → 72 °C, 30 s; G3PDH, 94 °C, 30 s → 54 °C, 30 s → 72 °C, 30 s.
RT-PCR products (cyclin D1:514 bp; TS:208 bp; TP:353 bp; DHFR:231 bp; GAPDH: 309 bp) were analyzed by 2% agarose gel electrophroesis and visualized by EB staining, densitometric scanning of the bands was carried on and relative amount of each gene mRNA expression was estimated by normalized to the G3PDH mRNA detected in the same sample.
Results were expressed as mean±SD and the Student’s t test was used for statistical analysis (two sides). P < 0.05 was taken as level of significance.
Dose-dependent inhibitory effects were caused by cyclin D1 ASODN in SGC7901 cells,as shown in Figure 1. At concentration of 0.2 µmol/L, the inhibitory rate was 54.7% and 0.2 µmol/L was taken as optimal concentration for further experiments. Transfection with 0.2 µmol/L cyclin D1 ASODN significantly inhibited the growth of cells. IC50 in ASODN group was lower than that in the control from the third day as shown by growth curves, and there was no difference between SODN group and the control (Figure 2).
After transfecting with cyclin D1 SODN or ASODN, cells were treated with 5-FU, MTX or CDDP in different concentrations. We found that transfection with cyclin D1 ASODN enhances the cytotoxicity of 5-FU, MTX and CDDP significantly, the IC50s in ASODN + chemotherapy groups to different chemotherapeutic agents were significantly lower than the those in chemotherapy group and SODN+ chemotherapy group. The dose-effect curves and IC50s to different treatment are shown in Figure 3 and Table 2.
Relative cyclin D1, TS, TP and DHFR mRNA expression is quantified by densitometric analysis, and illustrated in Figure 4. Comparing with the control, the cyclin D1 mRNA expression reduced to 26.3% and 50.0% at 24 h and 48 h, respectively. In comparison with the control, TS mRNA levels were reduced to 83.5% and 48.4% at 24 h and 48 h, respectively. TP mRNA levels were significantly increased to 124.6% and 148.3% at 24 h and 48 h, respectively. DHFR mRNA levels were reduced to 52.3% and 37.5% at 24 h and 48 h, respectively.
The use of antisense oligonucleotides as selective inhibitor of gene expression has become an important tool in current laboratory research and clinical trails. In our study ASODN were phosphorothioate modified and encapsulated by LipofectAMINE2000, to enhance the efficiency of transfection and prolong the time of action. We found dose-dependent inhibitory effects were caused by cyclin D1 ASODN in SGC7901 cells, the percentage of inhibition were 54.7% in cells treated with 0.2 μmol/L cyclin D1 ASODN for 24 h, and the growth of cells in ASODN group were also inhibited as shown by growth curves. Furthermore, cyclin D1 ASODN markedly reduced mRNA expression of cyclin D1. It suggested that cyclin D1 ASODN could inhibit the growth of gastric carcinoma cells through decreasing the cyclin D1 expression. The expression of cyclin D1 mRNA at 48h elevated a little, the lost of inhibitory effect of ASODN may be due to digestion by nucleases existed in cells.
5-fluoro-2’-deoxyuridine 5’-monophosphate (FdUMP), metabolic product of 5-FU, forms a tight-binding complex with TS and thereby blocks DNA synthesis process. Inhibition of TS by FdUMP is one of main mechanism underlying 5-FU action, the degree of inhibition of TS and the persistence of inhibition are essential factors for maximal in vivo growth inhibition by 5-FU[3]. TP is an enzyme that involved in pyrimidine nucleoside metabolism, and able to catalyze the conversion of 5-FU to 5-fluoro-2’-deoxyuridine (5-FdUR), which is the first step in one pathway for the metabolic activation of 5-FU. The 5-FdUR can be activated to 5-FdUMP, which blocks TS activity,and exerts antiproliferative effect.[4] The major target for MTX is the enzyme DHFR, which is important in biosynthesis of RNA and DNA. MTX has a chemical structure similar to folic acid, and can competitively inhibit DHFR activity. At 48 h after cyclin D1 ASODN transfection, the mRNA expression of TS, TP, DHFR in SGC7901 cells changed significantly in accompany with enhancement of chemosensitivity as shown in our study.
E2F may play an important role in this alteration. E2F is a cellular transcription factor, which can induce a number of genes important in the passage of cell cycle through the G1/S phase transition as well as in the initiation of DNA synthesis. When E2F binds the promoter and pRb simultaneously, the pRb-E2F complex acts as a transcriptional suppressor and completely silences the transcription of target genes. Interaction with E2F is the means through which pRb exerts its antiproliferative effect[5]. It would be expected that decreased expression of cyclin D1 by ASODN might affect pRb phosphorylation and might lead to change of level of E2F protein as a consequence of alteration in their interactions with pRb,thus change the expression of TS, TP and DHFR and enhance the chemosensitivity to 5-FU and MTX.
Warenius et al [6] firstly found that high cyclin D1 expression is related to CDDP resistance in the 16 human cell lines. Furthermore, CDDP resulted in significantly higher rates of cell killing in the antisense cyclin D1 transfected laryngeal squamous cell carcinoma cell lines CCL23 than parental cells, and ID50 decreased, which suggest the decreased expression of cyclin D1 may enhance the DNA-damaging effects of CDDP [7]. It is possible that the blockade of autocrine mitogenic signals by inhibiting cyclin D1 expression might also be a mechanism for increase in CDDP chemosensiti-vity[8]. As shown in our study, cyclin D1 ASODN can increase the cheosensitivity to CDDP in SGC7901 cells, but the exact mechanism needs further research.
Cyclin D1 ASOND could inhibit the growth of gastric carcinoma cells and change the expression of enzymes related to metabolism of chemotherapeutic agents through the influence on expression of cell cycle regulators, and enhance the chemosensitivity to 5-FU, MTX and CDDP, indicating that ASODN technology is a feasible way to enhance the efficiency of chemotherapeutic agents and provide a new strategy for combination therapy of gastric cancer.
S- Editor Wang J L- Editor Zhang JZ E- Editor Zhang Y
| 1. | Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909-2915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 323] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Cagnoli M, Barbieri F, Bruzzo C, Alama A. Control of cyclin D1 expression by antisense oligonucleotides in three ovarian cancer cell lines. Gynecol Oncol. 1998;70:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3255] [Cited by in RCA: 3619] [Article Influence: 164.5] [Reference Citation Analysis (0)] |
| 4. | La Thangue NB. The yin and yang of E2F-1: balancing life and death. Nat Cell Biol. 2003; 5: 587-589. Nat Cell Biol. 2003;5:655-660. [PubMed] |
| 5. | Bell LA, Ryan KM. Life and death decisions by E2F-1. Cell Death Differ. 2004;11:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Warenius HM, Seabra LA, Maw P. Sensitivity to cis-diamminedichloroplatinum in human cancer cells is related to expression of cyclin D1 but not c-raf-1 protein. Int J Cancer. 1996;67:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wang MB, Yip HT, Srivatsan ES. Antisense cyclin D1 enhances sensitivity of head and neck cancer cells to cisplatin. Laryngoscope. 2001;111:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Kornmann M, Danenberg KD, Arber N, Beger HG, Danenberg PV, Korc M. Inhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genes. Cancer Res. 1999;59:3505-3511. [PubMed] |