Published online Mar 7, 2005. doi: 10.3748/wjg.v11.i9.1287
Revised: August 21, 2004
Accepted: November 19, 2004
Published online: March 7, 2005
AIM: To investigate the correlation between microvessel density and spiral CT perfusion imaging in colorectal carcinoma.
METHODS: Thirty-seven patients, with histologically proven colorectal carcinoma, underwent water enema spiral CT scan. The largest axial surface of the primary tumor was searched on unenhanced spiral CT images. At this level, the enhanced dynamic scan series was acquired. Time-density curves (TDC) were created from the region of interest drawn over the tumor, target artery by Toshiba Xpress/SX spiral CT with perfusion functional software. Then the perfusion was calculated. Microvessel density (MVD) was evaluated using immunohistochemical staining of surgical specimens with anti-CD34, and then MVD was correlated with perfusion.
RESULTS: MVD of colorectal carcinomas was 33.11-173.44, mean 87.28, and perfusion was 15.60-64.80 mL/min/ 100 g, mean 39.74 mL/min/100 g. MVD and perfusion were not associated with invasive depth, metastasis and disease stage, and they all decreased with increasing Dukes’ stage, but no significant correlation was found between them (r = 0.18, P = 0.29).
CONCLUSION: There is no significant correlation between MVD and perfusion. Neovascularizaton and perfusion are highly presented in early colorectal carcinoma. CT perfusion imaging may be more suited for assessing tumorigenesis in colorectal carcinoma than histological MVD technique.
- Citation: Li ZP, Meng QF, Sun CH, Xu DS, Fan M, Yang XF, Chen DY. Tumor angiogenesis and dynamic CT in colorectal carcinoma: Radiologic-pathologic correlation. World J Gastroenterol 2005; 11(9): 1287-1291
- URL: https://www.wjgnet.com/1007-9327/full/v11/i9/1287.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i9.1287
Microcirculation as a result of angiogenesis plays an important role in the growth, metastasis, detection, and treatment of tumors. The histological microvessel density (MVD) technique is the current gold standard to characterize tumor angiogenesis[1]. Several studies have shown a statistically significant correlation between MVD and invasion and metastasis in various solid tumors such as breast, lung, uterine cervix, and prostate. However, this relationship is controversial in colorectal carcinoma (CRC)[1,2]. Spiral CT (SCT) perfusion imaging has been used to assess the microcirculatory change of tumor and to indirectly reflect the tumor angiogenesis[3]. To our knowledge, no study has been performed on the relationship between MVD and SCT perfusion imaging. Therefore, the aim of the present study is to evaluate the correlation between MVD and the perfusion estimated from dynamic CT examination.
A total of 37 patients with CRC (19 men, 18 women; age range 24-87 years; mean age 54.1 years), who had undergone colectomy at our institution, were included in this study. Patients were selected according to the following criteria: (1) no pre-operative treatment such as chemotherapy, radiotherapy; (2) absence of contraindications to the administration of contrast medium; (3) probable ability to cooperate with the whole procedure, and (4) without severe disease in heart, liver, lung, and kidney. The time between CT examinations and surgery ranged from 2 to 13 d, with a mean time of 6.4 d. All of them proved to have adenocarcinomas. Among them, one patient had well-differentiated adenocarcinoma, 33 patients had moderately differentiated adenocarcinoma, and three patients had poorly differentiated adenocarcinoma. They were staged according to Dukes’ classification. The pathologic diagnoses of CRC included eight patients with stage A disease, 16 patients with stage B disease, 11 patients with stage C disease, two patients with stage D disease. The tumors located in rectum in 28 patients, in sigmoid colon in six patients, in descending colon in one patient, in ascending colon in two patients.
Before the examination began, patients were carefully instructed in and practised the breath-holding technique to reproduce precisely the same degree of inhalation or exhalation for each scan series. One day before CT, all patients received colon-cleansing preparation. Immediately before CT, patients were positioned on the CT table, a barium enema tube inserted into the rectum. Subsequently, <2000 mL lukewarm water was administered per rectum to distend the colon. The enema was stopped whenever the patients complained of discomfort.
Single-location dynamic scans were obtained at the selected section with use of a Toshiba Xpress/SX spiral CT. Based on the clinical information, the largest axial surface of the primary tumor was searched on unenhanced CT images. At this level, the enhanced dynamic scans were acquired. A 50 mL intravenous bolus of a nonionic iodinated contrast medium (iopromide, Ultravist 300; Schering, Berlin, Germany) was administered via the antecubital vein at a rate of 5 mL/s by using an autoinjector. Three series of CT scans (10 scans each for the first and second series and 15 scans for the third series) were obtained beginning at 7, 40, and 76 s after injection of contrast medium. For the first series, the scanning interval was 2 s; for the second series, the scanning interval was 3 s; and for the third series, the scanning interval was 6 s. Thirty-five contrast enhanced dynamic images were obtained in each patient. CT scanning parameters were: section thickness of 5 mm, 320×320 mm field of view, 180 mA, 120 kV, and a 512×512 matrix.
Time density curves (TDC) were created from regions of the interest drawn over the tumor, target artery (abdominal aorta, external iliac artery, or femoral artery) by Toshiba Xpress/SX spiral CT with perfusion functional software. The region of interest (ROI) was placed in the maximally enhanced area of the tumor and as large as possible to minimize noise but with care to avert partialvolume effect. Tumoral perfusion (PF) was calculated by applying a nuclear medicine data processing technique to the time density data; this technique was based on the general equation as described by Miles et al[4]:
PF = [dCtumor/dt(max)]/[Cartery(max)] ×60×100 (Unit: mL/min/100 g).
Where dCtumor/dt(max) is the maximum slope of TDC as measured in the tumor, and Cartery(max) is given as the peak enhancement within a blood vessel contained in the slice studied. The size, morphology and exact location of tumor at the selected image were recorded.
Histopathologic examination was performed in all patients using pathologic specimens in the planes that correspond to ROI of the CT imaging sections (Figure 1). Tumor tissue specimens were fixed with 40 g/L formaldehyde and embedded in paraffin.
The sections were incubated in an anti-CD34 monoclonal antibody (Maxin Biotechology, Inc., Fuzhou). Criteria for vessel counting were those established by Weidner et al[5]. Any single brown-staining endothelial cell or small clusters of brown-staining endothelial cells, with or without a lumen, clearly separate from adjacent microvessels, tumor cells, and other connective tissue elements were considered as individual vessels. Vessels of a caliber larger than approximately eight red blood cells and vessels with a thick muscular wall were excluded from the final count. The microvessel count was performed in all cases by a single pathologist.
Slides were examined at low-power magnification (×40) to identify the areas with the highest density of microvessels. In each case, the three most vascularized areas were selected and the three fields of the microvessels in a ×200 field of these three areas were counted. The average counts of the nine ×200 fields were recorded for analysis.
Data were expressed as mean±SD. The relationship between MVD, PF and the various clinicopathological factors was examined by Student’s t test. One-way ANOVA was used to test the correlation among different Dukes’ stages. A Pearson correlation coefficient test was conducted to compare MVD with PF. All statistical analyses were performed using SPSS statistical software. P<0.05 was considered significant.
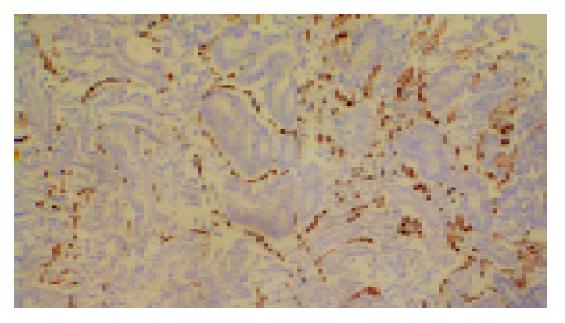
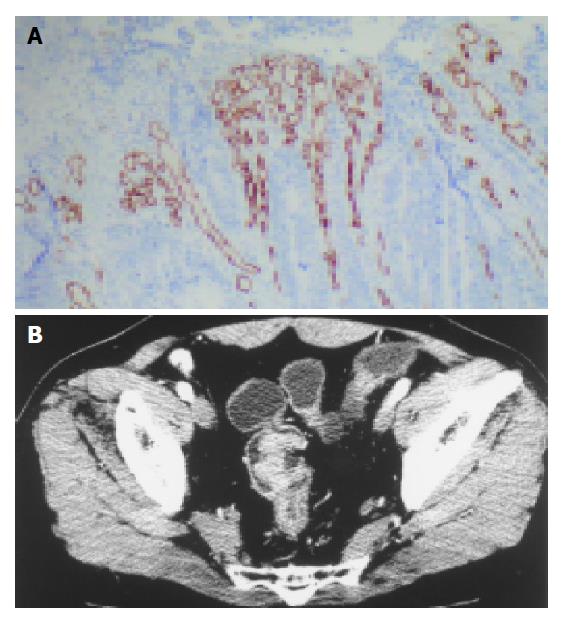
The microvessels in CRC labeled by anti-CD34 represented single endothelial cell or small clusters of endothelial cells, with or without an irregular lumen. The size and morphology of vessels were variable. The distribution of angiogenesis in a tumor was uneven and heterogeneous, and the difference of MVD between the two areas could reach 200 within the same section (Figure 2). In some cases MVD was very low, however, large vessels were abundant (Figure 3A).
MVD of the 37 colorectal cancers were 87.28±41.18, ranged from 33.11 to 173.44. No differences in MVD were demonstrated among different stages (F = 0.40, P = 0.67). MVD appeared to decrease with increasing Dukes’ stage. There was no statistically significant association between MVD and clinicopathologic factors such as invasion and lymph nodal metastasis (t = 0.69, 0.62, respectively, P = 0.50, 0.43, respectively) (Table 1).
| Variables | Patients | MVD | PF |
| Dukes’ stage1 | |||
| Stage A | 8 | 98.89±38.76 | 47.33±8.49 |
| Stage B | 16 | 81.89±45.33 | 38.06±7.44 |
| Stage C | 11 | 87.73±38.38 | 36.66±14.26 |
| Wall invasion | |||
| Through the wall | 11 | 95.17±34.41 | 43.31±9.96 |
| Within the wall | 26 | 84.44±42.48 | 37.73±10.80 |
| Lymph node metastasis | |||
| Negative | 24 | 87.06±43.30 | 41.15±8.83 |
| Positive | 13 | 87.22±36.43 | 36.14±13.38 |
PF in CRC was 39.74±10.82 mL/min/100 g, ranged from 15.62 to 64.80 mL/min/100 g. There was no significant correlation between PF and clinicopathologic factors as tested in this study (P = 0.07, 0.15, 0.18, respectively) (Table 1). PF tended to decrease with increasing Dukes’ stage (Figure 4).
MVD was not correlated to PF (r = 0.18, P = 0.29). Tumors with low PF (<40 mL/min/100 g) and high PF (>40 mL/min/100 g) were compared. The MVD of the group with high PF and that with low PF were not significantly different (Table 2). In some cases low MVD was associated with a high PF (Figure 3B).
| PF | Patients | MVD |
| >40 | 20 | 94.65±45.89 |
| <40 | 17 | 77.91±33.48 |
| T | 1.21 | |
| P | 0.2 |
In CRC, the correlation between MVD and clinicopathologic factors has been controversial[2,6,7]. Takebayashi et al[8] evaluated angiogenesis in 166 colorectal carcinomas and reported a correlation between MVD and depth of invasion and lymph node metastasis. The studies by Bossi et al[2] and Chen et al[7] as well as our present study did not show any significant association. Potential explanations for such differences included the grade of CRC examined, the endothelial cell marker used, and field magnification under which the vessels were counted[7]. Otherwise, the invasion and metastatic process depended on several factors, including angiogenesis. Recent study has shown that another microvascular channels termed ‘vasculogenic mimicry’ existed in tumors[6]. These vascular channels were generated by aggressive tumor cells or tumor stroma other than endothelial cells. The panendothelial antibodies may not demonstrate this type of neovascularization. Vasculogenic mimicry was closely correlated with invasiveness and metastasis of tumors. Some reports failed to show a relationship between MVD and lymph nodal metastasis and outcome in patients with malignant melanoma and in patients with squamous carcinoma of the tongue. They implied that tumor growth and spread may be facilitated by pre-existing vessels in highly vascular organs such as tongue, liver, kidney, or gastrointestinal tract[5]. Additional studies will be needed to further clarify the levels of MVD in colon tumorigenesis and tumor progression, as well as its clinical significance.
MVD was related to invasion and metastasis in several types of carcinoma, including breast, lung and prostate, it appeared that angiogenesis was progressively stimulated during tumor progression[2]. White et al[9] reported that MVD in CRC was significantly increased compared to adenomas and normal colonic mucosa. However, MVD was not related to Dukes’ stage and appeared to decrease slightly with increasing Dukes’ stage, as the results of Bossi et al[2] and our present study. These data suggested that angiogenesis in CRC was quite different from other organ tumorigenesis and neovascularization were highly presented in early CRC. Angiogenesis in CRC was equally stimulated in all pathologic disease stages. These findings implied that even small, superficially invasive carcinomas were capable of eliciting neovascularization of the same extent as seen in tumors presenting with distant metastasis.
In this study, PF of CRC was not correlated with tumor stages, invasion depth, and lymph node metastasis similar to the reports by Chen et al[10] in gastric cancer with color Doppler vascularity index. However, our study showed that PF appeared to decrease with increasing Dukes’ stage, as the same trend in MVD, but no significant correlation was found between PF and MVD. High perfusion was presented in early CRC. We found that MVD was not always consistent with PF, which was also revealed in other studies[11]. Sometimes high MVD was associated with a low PF. The reasons probably were: (1) In the process of tumorigenesis, vascular endothelial cells may be proliferated to either generate or not the functional vessels with lumen. However, only the functional or perfusion vessels would be reflected by CT perfusion imaging[12]; (2) The intratumoral interstitial hypertension increased with the development of the tumor vasculature, partial or total collapse of vessels may contribute to a low perfusion[13]. Sometimes low MVD was associated with a high PF. The reasons probably were: (1) CD34 antibodies could not labeled all vascular endothelial cells and therefore, MVD may be underestimated[5], (2) The size of CD34-positive microvessels was 0.02-0.10 mm[14] However, large vessels were abundant in some cases with rather low MVD and were associated with high perfusion (Figure 3); (3) ROI contained more areas with the highest density of microvessels than examined part of tissue section did; (4) The established arteriovenous shunts facilitated the passage of blood from artery to venule without passage through the microvessels[15].
The histological MVD technique may not be an ideal tool for clinical purposes, because it is invasive, prone to sampling errors and does not assess the functional angiogenic activity. On the contrary, CT perfusion imaging can dynamically reflect microcirculatory function in living individual. It is non-invasive, and can be repeated frequently. Some studies reported that perfusion estimated from CT or MRI may be of value in assessing the response to chemotherapy or radiotherapy, predicting the clinical outcome, and differentiating diseases[3,15,16]. CT perfusion imaging is an emerging technique with an increasing range of applications. Further studies are required to fully determine the clinical value of the technique in CRC.
In conclusion, there is no significant correlation between MVD and perfusion. Neovascularizaton and perfusion are highly presented in early CRC. CT perfusion imaging may be more suited for assessing tumorigenesis in CRC than the histological MVD technique.
Assistant Editor Guo SY Edited by Gabbe M
| 1. | Hawighorst H, Knapstein PG, Knopp MV, Weikel W, Brix G, Zuna I, Schönberg SO, Essig M, Vaupel P, van Kaick G. Uterine cervical carcinoma: comparison of standard and pharmacokinetic analysis of time-intensity curves for assessment of tumor angiogenesis and patient survival. Cancer Res. 1998;58:3598-3602. [PubMed] |
| 2. | Bossi P, Viale G, Lee AK, Alfano R, Coggi G, Bosari S. Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res. 1995;55:5049-5053. [PubMed] |
| 3. | Hermans R, Lambin P, Van der Goten A, Van den Bogaert W, Verbist B, Weltens C, Delaere PR. Tumoural perfusion as measured by dynamic computed tomography in head and neck carcinoma. Radiother Oncol. 1999;53:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Miles KA, Hayball MP, Dixon AK. Measurement of human pancreatic perfusion using dynamic computed tomography with perfusion imaging. Br J Radiol. 1995;68:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9-19. [PubMed] |
| 6. | Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 490] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 7. | Chen CN, Cheng YM, Liang JT, Lee PH, Hsieh FJ, Yuan RH, Wang SM, Chang MF, Chang KJ. Color Doppler vascularity index can predict distant metastasis and survival in colon cancer patients. Cancer Res. 2000;60:2892-2897. [PubMed] |
| 8. | Takebayashi Y, Aklyama S, Yamada K, Akiba S, Aikou T. Angiogenesis as an unfavorable prognostic factor in human colorectal carcinoma. Cancer. 1996;78:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, Murray JC. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002;62:1669-1675. [PubMed] |
| 10. | Chen CN, Cheng YM, Lin MT, Hsieh FJ, Lee PH, Chang KJ. Association of color Doppler vascularity index and microvessel density with survival in patients with gastric cancer. Ann Surg. 2002;235:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Pahernik S, Griebel J, Botzlar A, Gneiting T, Brandl M, Dellian M, Goetz AE. Quantitative imaging of tumour blood flow by contrast-enhanced magnetic resonance imaging. Br J Cancer. 2001;85:1655-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 617] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 13. | Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264-4266. [PubMed] |
| 14. | Tateishi U, Kusumoto M, Nishihara H, Nagashima K, Morikawa T, Moriyama N. Contrast-enhanced dynamic computed tomography for the evaluation of tumor angiogenesis in patients with lung carcinoma. Cancer. 2002;95:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | DeVries AF, Kremser C, Hein PA, Griebel J, Krezcy A, Ofner D, Pfeiffer KP, Lukas P, Judmaier W. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiology. 1997;205:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |