CASE REPORT
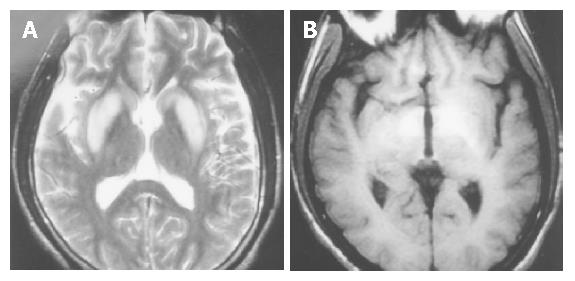
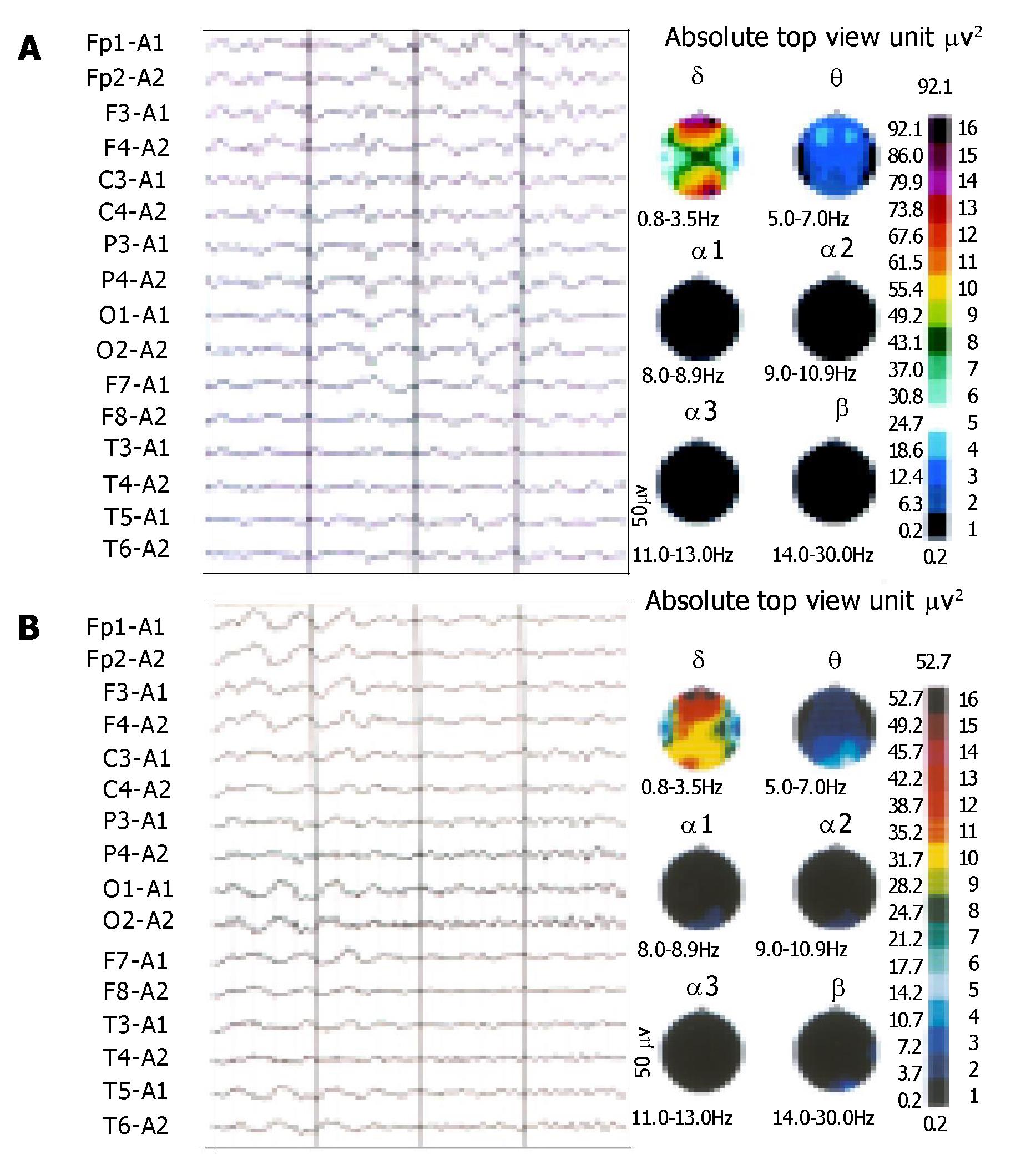
The patient was a 28-year-old man. He had a history of HBV-related liver cirrhosis. No familial hepatic or neurological diseases were reported. Two weeks ago, he had an episode of tary stool and was cured. But he developed apathy, dysarthria, mild consciousness impairment and involuntary abnormal movements after some days. Physical examination revealed splenomegaly and shifting dullness. Abdominal ultrasonic examination suggested portal hypertension and a great deal of ascites. Cranial magnetic resonance imaging (MRI) revealed increased signal intensity in basal ganglia bilaterally on T2-weighted images similar to hepatolenticular degeneration (Wilson’s disease) (Figure 1). Electroencephalogram (EEG) disclosed diffuse slow wave activity (Figure 2A). Slit lamp examination found no Kayser-Fleischer (K-F) ring of the cornea associated with Wilson’s disease. Copper balance and cerebrospinal fluid examinations gave normal results. Liver failure was grade B-9 of the Child-Pugh classification. The level of serum ammonia was measured several times, and was above the reference range (132 μmol/L) one time. He was treated with hepatic protector tablets, ammonia lowering agents, bowel relaxants and branched chain amino acid infusion. But therapeutic effect was not so good. Then naloxone and anexate (Flumazenil) were added, but the symptoms did not relieve obviously. Finally, after administration of biocoene (coenzyme complex injection), his neuropsychiatric abnormalities and the presentation of EEG markedly improved (Figure 2B). By 3 mo follow-up, we found that he recovered well and could take care of himself after leaving hospital.
Figure 1 Cranial MRI in the patient with similar hepatolenticular degeneration (Wilson’s disease).
A: increased signal intensity in basal ganglia bilaterally on T2WI; B: increased signal intensity in basal ganglia bilaterally on T1WI.
Figure 2 θ wave intermixed with δ wave, triphasic wave and spike and ware wave on EEG before treatmant(A).
and waves reduction on EEG after treatmant(B).
DISCUSSION
AHD is an exceptional type of hepatic encephalopathies (HE). It has been described in patients with severe liver disease of many causes, and notably in patients with surgically or spontaneously induced porto-systemic shunts. But many questions remain unanswered. Its frequency and pathogenesis remain largely uncertain. The clinical, neuroradiological, and biological characteristics of AHD have not yet been fully determined. The response to treatment is also incompletely understood. Our review of the literature (including MEDLINE, from 1981 to April 2003) yielded 36 similar cases[1-13], among them, 8 (22.2%) were Chinese and 28 (77.8%) were from Western countries.
Studies have indicated that the disease is associated with multiple metabolic insults, such as ammonia, manganese, etc. Especially, the toxic effects of manganese might be the major determinant[1-3,14]. It has been proved that manganese is cleared by the hepatobiliary system and whole blood and cerebrospinal fluid manganese concentrations in some patients with AHD are several fold above the reference range. Thas deposition of manganese in the brain is postulated in patients with AHD, which may induce diffuse degeneration in parenchymal brain. Microscopically, neuronal loss, Alzheimer type II astrocytes and cytoplasmic glycogen granules in basal ganglia are characteristic[3-6,14,15]. The spectrum of clinical presentations can be neuropsychiatric (apathy, lethargy, excessive somnolence, secondary dementia, etc.), extrapyramidal symptoms (focal dystonia, postural tremor, myoclonus, rigidity, dysarthria, choreoathetosis, etc.), or both[1-5,7-9,14]. But no consciousness impairment has been reported. MR imaging scans showed a hyperintense T1 signal in the basal ganglia[1-3]. But it was also reported that some patients showed increased signal intensity in the dentate nuclei bilaterally on T2-weighted images indistinguishable from Wilson’s disease. The clinical symptoms, neuropathological features and MR imaging appearance of AHD are rather uniform and similar to those seen in Wilson’s disease, so it is also named pseudo-Wilson disease. The discrimination depends on the following aspects: (1) Morbidity age: Wilson’s disease is a genetic disease, which rarely starts after the age of thirty, whereas AHD occurs in those with severe liver disease of many causes at different ages. (2) Copper metabolism: copper metabolism in vivo in Wilson’s disease is out of balance so that overload copper deposits in the liver, brain, kidney, cornea, etc. Thas the concentration of ceruloplasmin is under 200 g/L, the contents of copper in liver and urine are respectively over 250 μg per gram dry weight tissue and 100 μg/24 h. But copper metabolizes normally in patients with AHD. (3) Wilson’s disease is characterized by Kayser-Fleischer ring of the cornea as a result of abnormal copper deposition in the cornea. But this change is not seen in AHD[4,5,10]. Moreover, the disease is also distinct from the more acute and transient episodes of HE. The neurological symptoms of HE disappear when the disease relieves, and there is no organic damage in HE[11]. The disease develops gradually and the symptoms become progressively worse. Medical treatment is often disappointing. But it has been reported that some patients with AHD are responsive to branched chain amino acids or levodopa therapy. It has also been reported that endovascular occlusion of a porto-systemic shunt is temporarily effective. Moreover, liver transplantation in selected cases could be curative. is that AHD might be a reversible and treatable disorder partly[1,2,10,14].
As described above, the disease is characterized by neuropsychiatric and extrapyramidal symptoms developing from chronic hepatic encephalopathies. By review of similarly published cases at home and abroad, there has been no consciousness impairment reported. The case was exceptional for the presentation of mild consciousness impairment. Therefore, we hold that the symptoms of AHD might be overlapped partly with those of hepatic encephalopathies. The patient was irresponsive to the routine therapy of anti-hepatic encephalopathies (such as branched chain amino acids, etc.), but responsive to biocoene. The reason might be that biocoene contains glutathione, which can play the role in detoxicating and nourishing the liver.