Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.6968
Revised: March 23, 2005
Accepted: March 24, 2005
Published online: November 28, 2005
AIM: To induce the pancreatic duct cells into endocrine cells with a new natural protocol for electrophysiological study.
METHODS: The pancreatic duct cells of neonatal rats were isolated, cultured and induced into endocrine cells with 15% fetal bovine serum for a period of 20 d. During this period, insulin secretion, MTT value, and morphological change of neonatal and adult pancreatic islet cells were comparatively investigated. Pancreatic β-cells were identified by morphological and electrophysiological characteristics, while ATP sensitive potassium channels (KATP), voltage-dependent potassium channels (KV), and voltage-dependent calcium channels (KCA) in β-cells were identified by patch clamp technique.
RESULTS: After incubation with fetal bovine serum, the neonatal duct cells budded out, changed from duct-like cells into islet clusters. In the first 4 d, MTT value and insulin secretion increased slowly (MTT value from 0.024±0.003 to 0.028±0.003, insulin secretion from 2.6±0.6 to 3.1±0.8 mIU/L). Then MTT value and insulin secretion increased quickly from d 5 to d 10 (MTT value from 0.028±0.003 to 0.052±0.008, insulin secretion from 3.1±0.8 to 18.3±2.6 mIU/L), then reached high plateau (MTT value >0.052±0.008, insulin secretion >18.3±2.6 mIU/L). In contrast, for the isolated adult pancreatic islet cells, both insulin release and MTT value were stable in the first 4 d (MTT value from 0.029±0.01 to 0.031±0.011, insulin secretion from 13.9±3.1 to 14.3±3.3 mIU/L), but afterwards they reduced gradually (MTT value <0.031±0.011, insulin secretion <8.2±1.5 mIU/L), and the pancreatic islet cells became dispersed, broken or atrophied correspondingly. The differentiated neonatal cells were identified as pancreatic islet cells by dithizone staining method, and pancreatic β-cells were further identified by both morphological features and electrophysiological characteristics, i.e. the existence of recording currents from KATP, KV, and KCA.
CONCLUSION: Islet cells differentiated from neonatal pancreatic duct cells with the new natural protocol are more advantageous in performing patch clamp study over the isolated adult pancreatic islet cells.
- Citation: Leng SH, Lu FE. Induction of pancreatic duct cells of neonatal rats into insulin-producing cells with fetal bovine serum: A natural protocol and its use for patch clamp experiments. World J Gastroenterol 2005; 11(44): 6968-6974
- URL: https://www.wjgnet.com/1007-9327/full/v11/i44/6968.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i44.6968
The pancreatic islets (containing α, β, and δ cells that produce glucagon, insulin, and somatostatin, respectively) are intensively involved in the regulation of physiological metabolic homeostasis[1]. Patch clamp technique is considered as a very important approach for studying the endocrine activities and mechanisms of these cells. The traditional method for the isolation of rat intact pancreatic islet cells[2] has been performed for almost 40 years, and is still the most widely acceptable method in electrophysiological study of pancreatic islet cells. Nevertheless, pancreatic islet cells usually survive poorly after isolation because the cells are fragile in the in vitro culture condition[3]. Thus, only the freshly isolated or shortly cultured islet cells can be introduced for patch clamp experiments. Furthermore, there are some disadvantages in performing patch clamp experiments with isolated islet cells. The endocrine pancreas is considered as a slowly and continuously renewing tissue in the kinetic balance process of apoptosis and neogenesis[4]. Thus, pancreatic β cells isolated from adult rats are heterogeneous at different ages, including neonatal, adult, and senile cells. The isolated adult and senile β cells are more susceptible than neonatal β cells to the transition from the native living surroundings to the in vitro culture. Because the membrane of isolated adult β cells is fragile, it is difficult to manipulate gigaseal, a key step in patch clamp experiments.
Although there are other cell sources for performing patch clamp experiments without obvious problems mentioned above, such as β cell line and islet cells differentiated from marrow mesenchymal stem cells[5] or multipotent cells, and precursor cells of fetal pancreas, they still show considerable shortcomings. According to the reports, the β cell line only retains partial characteristics of primitive pancreatic islet cells, while the islet cells differentiated from marrow mesenchymal stem cells[6] and precursor cells of fetal pancreas[7-11] exhibit poor insulin secretory response to glucose. Therefore, it is essential to seek for more optimal cell sources and approaches suitable for patch clamp experiments.
Neonatal pancreatic duct cells are composed mainly of precursor cells[12,13] and can be induced into endocrine cells if appropriate morphogen stimuli are provided[10-13], and the induced endocrine cells are stable in culture condition[12,13], and their glucose-induced insulin secretion is evident[7-10]. Thus, we suppose that the neonatal pancreatic duct cells might be more suitable for patch clamp experiments.
Here the pancreatic duct cells were differentiated into endocrine islet cells with fetal bovine serum, a physiological nutrition[14]. The cell proliferation and insulin secretion of differentiated neonatal endocrine cells were compared to isolated adult endocrine islet cells. ATP sensitive potassium channels (KATP), voltage-dependent potassium channels (KV) and voltage-dependent calcium channels (KCA) of β cells were identified with patch clamp technique to prove the usefulness of this protocol.
Na2-ATP, EGTA, HEPES, repaglinide, and diazoxide were purchased from Sigma Co. The other chemicals used were of analytical reagent grade.
Sprague-Dawley rats aged 1-3 and 50-60 d were obtained from Laboratory Animal Center, Tongji Medical College, Huazhong University of Science and Technology. Hubei Experimental Animal Association approved the experiments. The culture medium used for both neonatal and adult cells was RPMI 1640 medium (glucose 11.1 mmol/L) supplemented with 15% fetal bovine serum, 15 mmol/L HEPES buffer, pH 7.40, without any antibiotics (to eliminate its possible influence on the differentiation of precursor cells). The cells were cultured at 37 °C in the atmosphere containing 950 mL/L O2+50 mL/L CO2. The isolation of pancreatic islet cells from adult rats was performed as previously described[2]. The isolation and induction of duct cells were performed as follows: the pancreases were moved from 10 neonatal rats and thoroughly washed in KRBH solution containing (in mmol/L) 136 NaCl, 4.8 KCl, 1 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 5 NaHCO3, 25 HEPES, 1% BSA, pH 7.40. The pancreases were minced into pieces of 1-2 mm in size. The minced pancreases were hand-shaken while being digested by 0.5 mg/mL type V collagenase in PBS (pH 7.40) for about 3-8 min in a 10-mL tube bathed in 37 °Cwater. Then digestion was stopped by 4 °C KRBH. The production was centrifuged (50 r/min) thrice to eliminate collagenase and broken cells. Thereafter, the cells were suspended in the cell culture medium and seeded in plastic dishes (18 mm in diameter) with 2 mL culture medium in each dish. After being cultured for about 24 h, the culture medium was moved, and the duct cells were washed with KRBH solution five times to remove the floated cells thoroughly. Then the left cells were mostly duct cells (Figure 1). The cells were kept in the cell culture medium for 20 d, during which the dishes were replenished with a new medium every 2 d.
The incubated cells were observed under inverted microscope each day. When the marked morphologic changes were observed, photograph of the cells was taken by phase difference microscopy.
All the isolated products from neonatal duct cells or adult pancreatic islet cells were equally seeded in gelatin-coated dishes (18 mm in diameter), and each dish contained 2 mL isolated products (about 104 cells). The supernatant of culture media in the five dishes was harvested every 2 d and kept at -20 °C for insulin determination by radioimmunoassay. The dishes were washed with KRBH solution twice, then 2 mL of the cell culture medium was added again for further culture.
MTT assay was carried out as previously described[15]. The isolated products from neonatal or adult rats (about 105 cells) were equally seeded in 10 dishes (96 cells in each dish, and each cell containing 150 μL isolated products), one dish of cells was used every 2 d for MTT determination, and the remaining dishes were replenished with a new medium.
The pancreatic islet cells were identified by dithizone staining method[16]. The viable cells were determined by trypan blue exclusive test.
Two steps were performed to identify pancreatic β cells from α and δ cells[17]. Therefore, only large cells with a capacitance of >5 pF were selected as β cells. Then a depolarizing protocol was applied to the large cells to identify the properties of voltage-dependent Na+ currents[18]. Thus, cells in which a Na+ current could be activated by a small depolarizing pulse from a prolonged holding potential of -70 mV were discarded. By contrast, cells exhibiting a Na+ current only after a hyperpolarizing pulse to -140 mV were considered to be β cells[19].
Patch clamp experiments were performed on β cells of isolated islet cells only in the first 4 d after the isolation, the β cells beyond this period of time could not be introduced into patch clamp experiments. The β cells differentiated from islet cells of neonatal rats were applied for patch clamp experiments from d 10 to d 16.
Rat pancreatic islet cells plated on the plastic dishes were placed in a recording chamber mounted on an inverted microscope (Olympus). Patch clamp recordings were done for cells that were not in contact with the other cells to avoid possible cell-cell coupling artifacts. For recording KATP currents, the solutions were as follows (in mmol/L): internal solution containing 140 KCl, 2 CaCl2, 4 MgCl2, 10 EGTA, 0.65 Na2-ATP, and 20 HEPES (pH 7.15 with KOH); and external solution containing 140 NaCl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2, 0.5 glucose and 10 HEPES (pH 7.4 with KOH). KATP currents were measured at a holding potential of -70 to -80 mV and -60 mV at 15-s intervals, and diazoxide (100 μmol/L) and repaglinide (100 μmol/L) were added respectively to further confirm whether it was KATP currents or not. For KV currents recording, the solutions were the same as for recording KATP currents except that 100 µmol/L repaglinide was added into external solution to block KATP currents. The cells were held at -120 mV and depolarized at 15-s intervals to 30 mV to activate KV currents. For KCA recording, the solutions were as follows (in mmol/L): internal solution containing 70 CsCl, 1 MgCl2, 4 ATP, 20 HEPES, 5 EGTA, pH 7.2; external solution containing 90 NaCl, 1 MgCl2, 20 TEA-Cl, 10 HEPES, 20 BaCl2, 5.6 KCl, 5 CsCl, pH 7.15. All cell membrane currents were measured using whole-cell mode of patch clamp technique at 22 °C, using an EPC-9 patch clamp amplifier (Heka Electronics, Lambrecht/Pfalz, Germany) and the software Pulsefit. Patch clamp electrodes were made from borosilicate glass capillaries (Shanghai Physiology Institute, China) using a two-stage puller (PP-830, Narishige Co., Japan) to give a resistance of 4-5 MΩ.
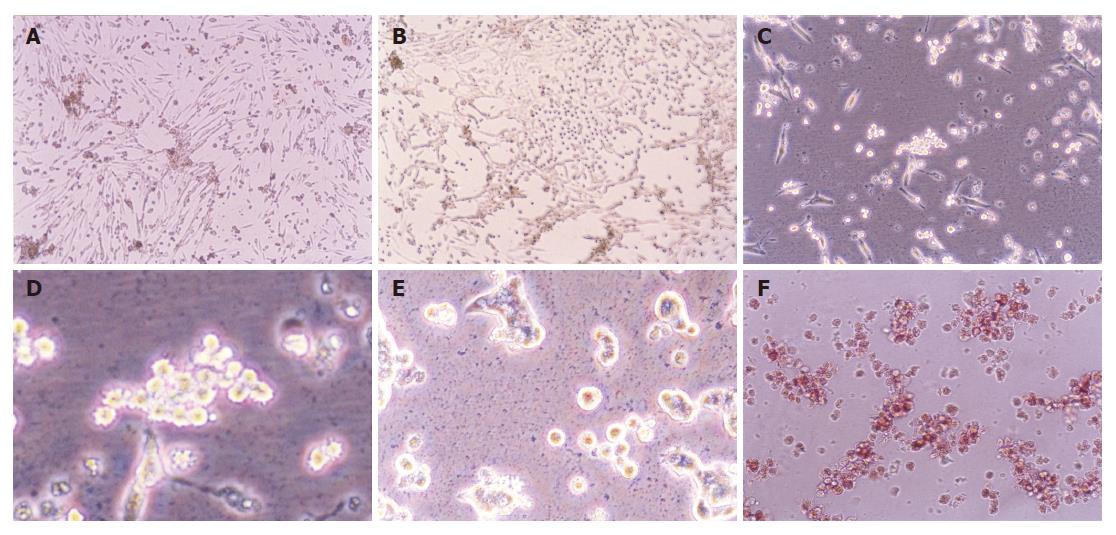
The neonatal rat pancreatic duct cells were organized in duct-like cells when incubated for 3-4 d (Figure 1A). There were precursors of pancreatic islet cells among them. When cultured for 5-10 d, some duct cells changed into round cells organized as islet clusters, being similar to pancreatic islet cells in vivo (Figure 1B). Then the cells grew bigger and budded out (Figures 1C and 1D). The buds grew bigger and bigger and became new ground cells in clusters (Figure 1E), which were further identified as pancreatic islet cells by dithizone (Figure 1F), a chemical considered to selectively stain pancreatic islet cells[16].
In contrast, the freshly isolated pancreatic islet cells from adult rats were round, but gradually became dispersed, broken or atrophied. After being cultured for a week, the islet cells were dispersed to be single cells, and the cell number was greatly reduced (data not shown).
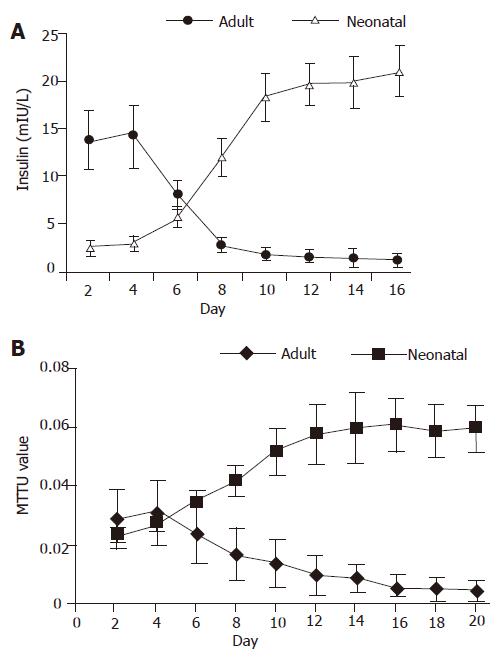
For neonatal pancreatic islet cells, the insulin concentration in the cell culture medium was low (from 2.6±0.6 to 3.1±0.8 mIU/L) in the first 4 d, then quickly increased (from 3.1±0.8 to 12.1±2.1 mIU/L ) from d 5 to d 10. Thereafter, the insulin release reached its high plateau (>18.3±2.6 mIU/L). For isolated pancreatic islet cells from adult rats, the insulin concentration was high (from 13.9±3.1 to 14.3±3.3 mIU/L) in the first 4 d, and gradually reduced (from 8.2±1.5 to 2.9±0.7 mIU/L) from d 5 to d 10. Then the insulin release was very low (<2.9±0.7 mIU/L) (Figure 2A).
For neonatal pancreatic islet cells, the MTT value was low (0.024±0.003 to 0.028±0.003) in the first 4 d and gradually increased (0.028±0.003 to 0.052±0.008) from d 5 to d 10. Thereafter, the MTT value reached its high plateau (>0.052±0.008). For isolated pancreatic islet cells from adult rats, the MTT value was stable (from 0.029±0.01 to 0.031±0.011) in the first 4 d and gradually reduced (0.031±0.01 to 0.014±0.008) from d 5 to d 10. Then the MTT value was very low (<0.014±0.008) (Figure 2B).
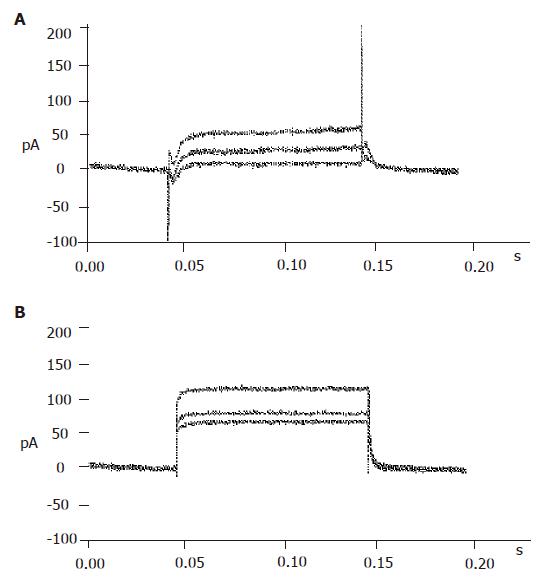
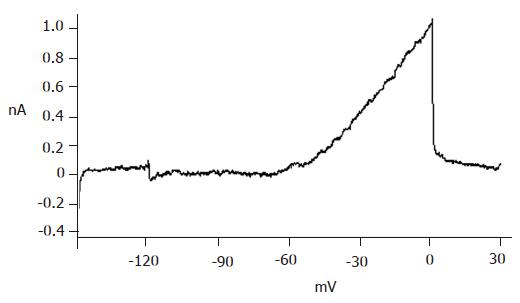
The big round cells (with a diameter >10 µm) were selected and their capacitance was determined (>5 pF). Then a depolarizing protocol was used to identify the properties of voltage-dependent Na+ currents. The results showed that voltage-dependent Na+ currents of selected cells could not be recorded at a holding potential of -70 mV (Figure 3B). On the contrary, the voltage-dependent Na+ currents of small round cells (capacitance <5 pF) could be detected at a holding potential of -70 mV (Figure 3A). It was demonstrated that the big round cells were pancreatic β cells, while small round cells were not.
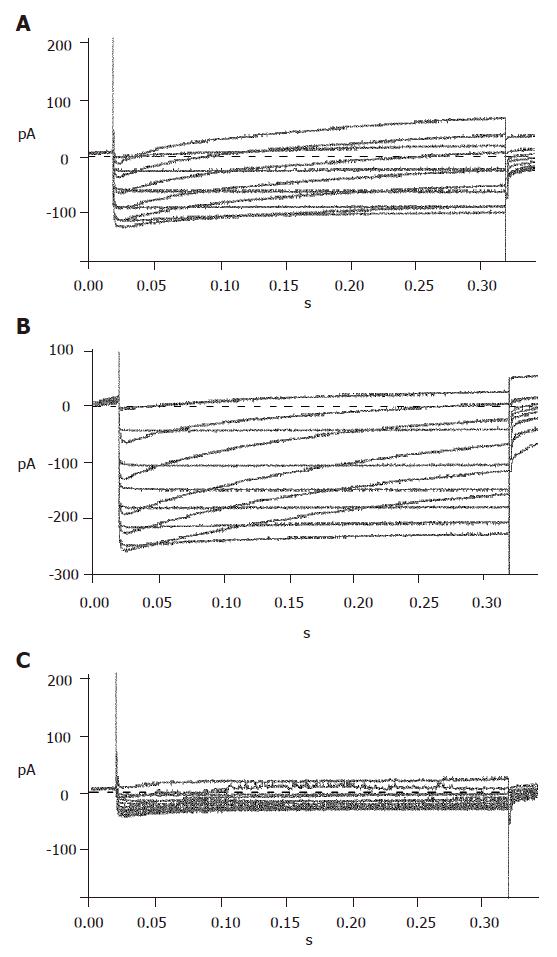
With certain internal and external solutions and protocol, the currents recorded in pancreatic β cells were almost entirely KATP currents[19]. To verify this, 100 μmol/L diazoxide, a special opener of KATP channels, and 100 μmol/L repaglinide, a special antagonist of KATP channels, were added correspondingly. The results showed that diazoxide enhanced, while repaglinide reduced the currents greatly (Figure 4), suggesting that the detected currents were from KATP channels.
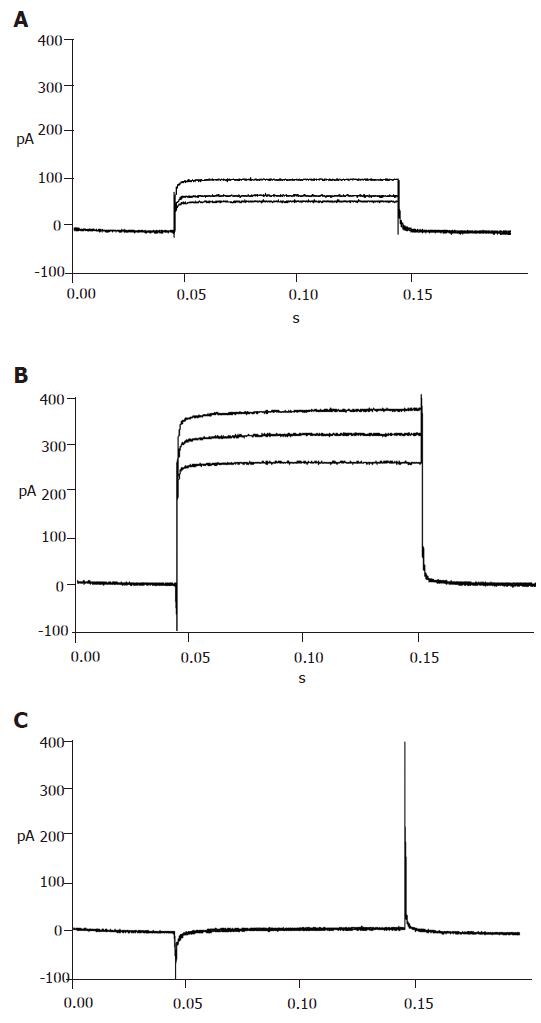
Repaglinide was used in the external solution to block KATP currents. Then the currents recorded should be almost entirely KV currents with the internal and external solutions and protocol. The recorded outward currents showed that the channels were inactive from -120 to -55 mV and active from -55 to 0 mV and the currents increased in proportion to voltage. When added with 100 µmol/L TEA, the currents could not be recorded, suggesting that the detected currents were from KV channels (Figure 5).
To efficiently record KCA currents, Cs+ was added in the internal solution, while TEA and Ba+ were added in the external solution to block voltage-dependent outward potassium currents. Meanwhile, Ba+ enhanced KCA currents. Inward currents were recorded at -40 mV and reached their peak at 0-10 mV, and then turned over at 50-60 mV. These dynamic electric patterns were characteristic of KCA currents[20,21]. Furthermore, special opener and antagonist of KCA channels were introduced to verify their existence. When added with 100 μmol/L Bay K8644, the currents enhanced greatly; while 100 μmol/L nifedipine was added, the currents reduced strikingly (Figure 6). These facts suggest that the detected currents were KCA currents (mainly of L-type calcium currents).
It has been reported that pancreatic endocrine cells of neonatal rats can be used in long-term study of secretion and electrophysiology[22]. According to the experimental protocol, fibroblasts are cleared away by timerosal to enhance insulin release. However, the enhancing effect of timerosal cannot be interpreted by the clearing away of fibroblasts, but might be produced due to the modified effects of timerosal on sulfonylurea receptor which regulates insulin release, because timerosal can modify mitochondrial sulfonylurea receptor[23]. Fibroblasts are present as the mesenchyme of islet cells, which may provide fibroblast growth factors and matrix to help the differentiation and replication of endocrine cells[24,25]. Therefore, alterations were made to optimize the experimental protocol correspondingly. Firstly, the fibroblasts in the pancreatic islet cells for the normal differentiation and replication of endocrine cells were retained. Secondly, timerosal was not introduced in our protocol to exclude its insulin regulating effect. Instead, fetal bovine serum, a physiological nutrition that does not intervene the normal neogenesis process, was administered in the cell incubation. With this natural protocol, the neonatal duct cells were successfully induced into endocrine cells.
It was reported that neonatal duct cells are composed mainly of precursor cells[12,13]. Thus, the harvested endocrine cells from neonatal rats should be mostly differentiated from precursor cells that lie in the pancreas duct tree. The harvested endocrine cells from the neonatal rats auto-organized as islet clusters (Figure 1B). Our observations that the islet cells changed from duct-like into round shape and that the round cells then budded outward in vitro culture, were in conformity with the report by Gershengorn et al[26].
Gigaseal, the key step in patch clamp experiments, is difficult to perform on isolated islet cells because the membrane is fragile and easily gets broken by the electrode. Even when gigaseal was completed, the currents recorded may be deformed or lower than normal because there are tiny gaps between cell membrane and the electrode, through which the currents might leak out. However, we found that it was easy to manipulate gigaseal on islet cells differentiated with this natural protocol. To explain this phenomenon, we compared the morphological and functional characteristics of neonatal and adult pancreatic islet cells in culture condition. For differentiated neonatal islet cells, insulin release was low during the early phase, but increased rapidly from d 5 to d 10, and then kept in high plateau (Figure 2). Correspondingly, the cell mass grew much bigger. As for isolated islet cells from adult rats, insulin release was high in the first 4 d, but then reduced quickly (Figure 2). Correspondingly, the islet cells became dispersed, broken or atrophied. For neonatal endocrine islet cells, the MTT value increased quickly from d 5 to d 10 and then kept in high plateau. For isolated islet cells from adult rats, the MTT value was stable in the first 4 d and then reduced quickly (Figure 3). The data are in conformity with the idea that isolated islet cells survive poorly in culture condition[3] and support that neonatal pancreatic islet cells are more preferable for long-term and stable culture, thus more suitable for patch clamp experiments.
It was reported that the differentiated neonatal pancreatic islet cells have evident glucose-induced insulin secretion property[7-10]. To understand its mechanism, we investigated the electric activities of KATP, KV, and KCA, regulators of glucose-induced insulin secretion process. The role of KATP channels of β cells in glucose-induced insulinotropic process has been well demonstrated[27]; the KCA and KV channels are also suggested to couple with glucose-dependent insulinotropic effects[28-32]. Thus, the properties of these channels in differentiated islet cells were studied. The fact that KATP, KV, and KCA channels in differentiated islet β cells are sensitive to corresponding antagonist and/or opener (Figures 4-6) indicates that the differentiated islet cells have evident glucose-induced insulin secretion properties.
In conclusion, the natural protocol illustrated in this paper enables the long-term incubation of stable endocrine islet cells, which will greatly optimize and facilitate the performance of patch clamp experiments and other single cell studies.
The authors thank Professor Yong-Jian Xu and technician Wang Ni (Respiratory Department of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology) for their help in patch clamp technique.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569-1580. [PubMed] |
| 2. | Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35-39. [PubMed] |
| 3. | Brandhorst D, Brandhorst H, Hering BJ, Bretzel RG. Long-term survival, morphology and in vitro function of isolated pig islets under different culture conditions. Transplantation. 1999;67:1533-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology. 2000;141:1926-1929. [PubMed] |
| 5. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 970] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 6. | Vogel G. Developmental biology. Stem cells are coaxed to produce insulin. Science. 2001;292:615-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Asplund K, Westman S, Hellerström C. Glucose stimulation of insulin secretion from the isolated pancreas of foetal and newborn rats. Diabetologia. 1969;5:260-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Asplund K. Dynamics of insulin release from the foetal and neonatal rat pancreas. Eur J Clin Invest. 1973;3:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Rhoten WB. Insulin secretory dynamics during development of rat pancreas. Am J Physiol. 1980;239:E57-E63. [PubMed] |
| 10. | Hole RL, Pian-Smith MC, Sharp GW. Development of the biphasic response to glucose in fetal and neonatal rat pancreas. Am J Physiol. 1988;254:E167-E174. [PubMed] |
| 11. | Tuch BE, Jones A, Turtle JR. Maturation of the response of human fetal pancreatic explants to glucose. Diabetologia. 1985;28:28-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97:2119-2129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 298] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Yoon KH, Quickel RR, Tatarkiewicz K, Ulrich TR, Hollister-Lock J, Trivedi N, Bonner-Weir S, Weir GC. Differentiation and expansion of beta cell mass in porcine neonatal pancreatic cell clusters transplanted into nude mice. Cell Transplant. 1999;8:673-689. [PubMed] |
| 14. | Hulinsky I, Hulinska H, Silink M. DNA synthesis in cultured neonatal rat islets--a comparison of two methods. Diabetes Res Clin Pract. 1995;27:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Janjic D, Wollheim CB. Islet cell metabolism is reflected by the MTT (tetrazolium) colorimetric assay. Diabetologia. 1992;35:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Fiedor P, Rowiński W, Licińska I, Mazurek AP, Hardy MA. The survival identification of pancreatic islets of Langerhans. In vitro and in vivo effects of two dithizone preparations on staining of rat and human islets of Langerhans-preliminary study (Part I). Acta Pol Pharm. 1995;52:431-436. [PubMed] |
| 17. | Göpel SO, Kanno T, Barg S, Rorsman P. Patch-clamp characterisation of somatostatin-secreting -cells in intact mouse pancreatic islets. J Physiol. 2000;528:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Plant TD. Na+ currents in cultured mouse pancreatic B-cells. Pflugers Arch. 1988;411:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Jonas JC, Plant TD, Henquin JC. Imidazoline antagonists of alpha 2-adrenoceptors increase insulin release in vitro by inhibiting ATP-sensitive K+ channels in pancreatic beta-cells. Br J Pharmacol. 1992;107:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Rorsman P, Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986;374:531-550. [PubMed] |
| 21. | Satin LS, Cook DL. Evidence for two calcium currents in insulin-secreting cells. Pflugers Arch. 1988;411:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Schwartz JL, Mealing GA, Whitfield JF, Braaten JT. Long-term culture of neonatal rat pancreatic endocrine cells as model for insulin-secretion and ion-channel studies. Diabetes. 1990;39:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Szewczyk A, Wójcik G, Lobanov NA, Nalecz MJ. Modification of the mitochondrial sulfonylurea receptor by thiol reagents. Biochem Biophys Res Commun. 1999;262:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Arany E, Hill DJ. Ontogeny of fibroblast growth factors in the early development of the rat endocrine pancreas. Pediatr Res. 2000;48:389-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Hulinsky I, Cooney S, Harrington J, Silink M. In vitro growth of neonatal rat islet cells is stimulated by adhesion to matrix. Horm Metab Res. 1995;27:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 319] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 27. | Schwanstecher C, Schwanstecher M. Nucleotide sensitivity of pancreatic ATP-sensitive potassium channels and type 2 diabetes. Diabetes. 2002;51 Suppl 3:S358-S362. [PubMed] |
| 28. | Rorsman P. The pancreatic beta-cell as a fuel sensor: an electrophysiologist's viewpoint. Diabetologia. 1997;40:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 198] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem. 1999;259:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | MacDonald PE, Sewing S, Wang J, Joseph JW, Smukler SR, Sakellaropoulos G, Wang J, Saleh MC, Chan CB, Tsushima RG. Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic beta-cells enhances glucose-dependent insulin secretion. J Biol Chem. 2002;277:44938-44945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | MacDonald PE, Salapatek AM, Wheeler MB. Glucagon-like peptide-1 receptor activation antagonizes voltage-dependent repolarizing K( ) currents in beta-cells: a possible glucose-dependent insulinotropic mechanism. Diabetes. 2002;51 Suppl 3:S443-447. |
| 32. | MacDonald PE, Wang G, Tsuk S, Dodo C, Kang Y, Tang L, Wheeler MB, Cattral MS, Lakey JR, Salapatek AM. Synaptosome-associated protein of 25 kilodaltons modulates Kv2.1 voltage-dependent K(+) channels in neuroendocrine islet beta-cells through an interaction with the channel N terminus. Mol Endocrinol. 2002;16:2452-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |