Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.6926
Revised: April 5, 2005
Accepted: April 8, 2005
Published online: November 28, 2005
AIM: To assess the functional status and etiology of liver cirrhosis by quantitative 31P magnetic resonance spectroscopy (MRS).
METHODS: A total of 80 patients with liver cirrhosis of different etiology and functional status described by Child-Pugh score were examined and compared to 11 healthy volunteers. MR examination was performed on a 1.5 T imager using a 1H/31P surface coil by the 2D chemical shift imaging technique. Absolute concentrations of phosphomonoesters (PME), phosphodiesters (PDE), inorganic phosphate (Pi) and adenosine triphosphate (ATP) were measured.
RESULTS: MRS changes reflected the degree of liver dysfunction in all the patients as well as in individual etiological groups. The most important change was a decrease of PDE. It was possible to distinguish alcoholic, viral and cholestatic etiologies based on MR spectra. Alcoholic and viral etiology differed in PDE (alcoholic, viral, controls: 6.5±2.3, 6.5±3.1, 10.8±2.7 mmol/L, P<0.001) and ATP (alcoholic, viral, controls: 2.9±0.8, 2.8±0.9, 3.7±1.0 mmol/L, P<0.01) from the control group. Unlike viral etiology, patients with alcoholic etiology also differed in Pi (alcoholic, controls: 1.2±0.4, 1.6±0.6 mmol/L, P<0.05) from controls. No significant changes were found in patients with cholestatic disease and controls; nevertheless, this group differed from both alcoholic and viral groups (cholestatic, alcoholic, viral: 9.4±2.7, 6.5±2.3, 6.5±3.1 mmol/L, P<0.005) in PDE.
CONCLUSION: 31P MRS can significantly help in non-invasive separation of different etiological groups leading to liver cirrhosis. In addition, MRS changes reflect functional liver injury.
- Citation: Dezortova M, Taimr P, Skoch A, Spicak J, Hajek M. Etiology and functional status of liver cirrhosis by 31P MR spectroscopy. World J Gastroenterol 2005; 11(44): 6926-6931
- URL: https://www.wjgnet.com/1007-9327/full/v11/i44/6926.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i44.6926
Cirrhosis is the final stage of various liver diseases. Regardless of etiology (i.e., viral, alcoholic, autoimmune, metabolic and others), the liver injury leads to the excessive accumulation of extracellular matrix and to nodular regeneration of parenchyma. Information about the etiology and degree of liver derangement is indispensable and usually needs to be verified by means of liver biopsy. This procedure is invasive, uncomfortable for the patient and sometimes not without serious complications. Therefore, efforts have been made to non-invasively obtain information concerning liver injury.
Additional important information is the degree of functional limitation of the liver. In clinical settings, this is usually described by a Child-Pugh score (CPS), which is calculated from clinical and laboratory tests[1-3]. As clinicians need to determine the specific etiological diagnosis of liver cirrhosis, all possible additional information is valuable in clinical practice to find an appropriate treatment[4]. Imaging examinations, which are capable of improving the diagnostic process, are very helpful.
One of the promising techniques is the application of magnetic resonance (MR) spectroscopy[5]. Phosphorus (31P) MR spectroscopy has been used to study liver metabolism in vivo for several years[6-9]. It enables the observation of energy metabolism and intracellular compartmentation through the signals of phosphomonoesters (PME), phosphodiesters (PDE), inorganic phosphate (Pi) and nucleotide triphosphates, mainly adenosine triphosphate (ATP). The PME and PDE signals are multicomponent with phosphorylcholine and phosphorylethanolamine which are the main contributors to PME as well as glycerophosphorylcholine and glycerophosphorylethanolamine which are the main contributors to PDE [10]. The final typical signal of 31P MR spectra in vivo is phosphocreatine (PCr). Although it is a dominant signal in muscles, it is not readily observable in spectra of the liver because of its low contribution to hepatic metabolic processes. Its presence indicates some contribution of signals from abdominal wall muscle as a partial volume effect.
In this study we investigated whether quantitative 31P MR spectroscopy can distinguish different etiologies of liver cirrhosis and assess the functional severity of liver injury. The main goal of the study was to describe the relationship between the concentration of phosphorylated metabolites in the liver and the different etiological groups of liver cirrhosis.
A group of 80 patients (49.7±11.5 years) with confirmed liver cirrhosis of different etiology (alcoholic cirrhosis in 33 cases, viral hepatitis in 22 cases (B virus in three cases, C virus in 17 cases, combined B+C in two cases), cholestatic liver disease in 16 cases (primary biliary cirrhosis in six cases, one case of secondary biliary cirrhosis, primary sclerosing cholangitis in eight cases and one case of biliary atresia), and other etiologies in nine cases (one Budd-Chiari, two congenital fibrosis, two autoimmune, and four cryptogenic cases) were examined. The last nine patients were excluded from etiological evaluations because of their etiological diversity and small number. Patients with combined etiologies (especially viral and alcoholic) were strictly excluded from the study as well as another 19 patients with insufficient resolution or low signal to noise ratio due to technical problems. Results were compared to those of a group of 11 healthy volunteers (40.5±10.9 years). Liver biopsy was performed in all cirrhotic patients except for those whose clinical, laboratory, endoscopic and imaging studies (abdominal ultrasound and CT) were typical and without any doubt for severe liver cirrhosis. Etiological diagnosis was made using standard diagnostic rules. All alcoholic patients admitted had a previous regular drinking of alcohol more than 80 g/d, patients with viral hepatitis B and C were confirmed by specific antibodies and/or PCR DNA/RNA positivity. Patients with primary biliary cirrhosis were AMA positive, patients with primary sclerosing cholangitis had typical cholangiography, all cholestatic patients were confirmed by liver biopsy. Similarly strict criteria were used for other etiologies. All patients were originally examined for the liver transplantation program and six-month alcohol abstinence in alcoholic patients was proved by independent observers, i.e. family members, primary care physicians and psychiatrists trained in substance abuse treatment. All subjects, including the healthy volunteers, abstained from alcohol during 48 h before MR examination and underwent standard clinical biochemical testing just before the MR examination which was performed in early morning after an overnight fast (at least 8 h of fasting). A Child-Pugh score [1-3] was obtained in all patients (mean CPS = 9.3) and patients were distributed into groups A, B, and/or C for statistical evaluation. MELD score was not used because of its main application in donor allocations. The subjects were fully informed and signed the protocol of the examination in accordance with rules approved by the ethical committee, which conform to the ethical guidelines of the 1975 Declaration of Helsinki.
MR examination was performed on a Siemens Vision (Erlangen, Germany) whole-body MR imager operating at 1.5 Tesla equipped with a commercial dual 1H/31P surface coil. The subjects were examined in a prone position with the liver centered on the surface coil. Neither ECG nor breathing monitoring due to this position was found to be necessary. No tremor because of encephalopathy which might also influence the quality of MR examination was observed. Basic MR images in all orientations were obtained for the localization of voxels (Figure 1). 31P MR spectra were measured using a standard two-dimensional chemical shift imaging (CSI) technique[5] in the transversal plane with the following parameters: TR = 323 ms, TE = 2.3 ms, matrix 16×16, field of view (FOV) = 480 mm, flip angle = 90º, slice thickness = 4 cm, voxel volumes were 3 cm×3 cm×4 cm, 12 acquisitions, acquisition time = 16 min.
Approximately 12 voxels (in the normal size of the liver) were selected from a whole CSI matrix (256 voxels). The most appropriate voxel for the quantitative evaluation[11] had to fulfill two conditions: (1) no visible PCr signal characterizing the presence of abdominal muscles; (2) no visible large intrahepatic blood vessels (portal vein truncus and its left and right lobar branches, inferior vena cava and large branches of hepatic veins). Such voxel was chosen using a standard postprocessing method (the movement of the whole CSI matrix) and considered as volume of interest (VOI) for quantitative evaluation. However, the contribution of small hepatic veins to measured signal intensities could not be excluded.
Spectra were evaluated using standard Siemens Numaris software (Gauss apodization with halfwidth = 30 ms, manual phase and spline baseline correction, Fourier transformation and curve fitting with the assumption of Gaussian line shapes). We used Pi chemical shift = 5 ppm as a standard frequency for the assignment of observed signals. Signal intensities of PME, Pi, PDE and βATP (αATP and γATP signals were not used for the evaluation because of overlap with signals of other compounds) were used for the measurement of absolute molar concentrations. The methodology of the absolute quantification using the CSI sequence was published previously[11,12]. The signal intensity ratios were not used because of the signal intensity dependence on the distance of the VOI from the center of the surface coil.
The comparison of several neighboring voxels from different places in the liver was performed and a VOI of 36 mL was found to be large enough to disregard structural heterogeneities. All data represented three independent evaluations and were expressed as mean±SD unless otherwise indicated.
Statistical analysis was performed using paired t tests and the technique of "contrast analysis" in the ANOVA module of STATISTICA 6[13] for multiple comparisons. Pattern recognition analysis of data was performed by principal component analysis (PCA) using the Multivariate Explanatory Techniques module of the STATISTICA software and by linear and nonlinear discriminant analyses (LDA and NDA, respectively)[14] using MaZda B11[15].
A Levene's test of homogeneity of variances in groups confirmed that there was no effect at P<0.05. This means data had the same variance and could be compared by standard t test. The null hypothesis H0 (group means are not different from the control group) was rejected if P<0.05 (5 % error level).
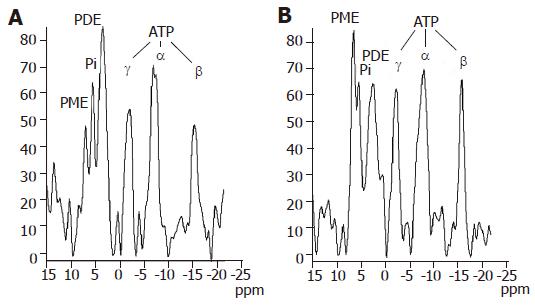
Figure 2 shows typical examples of phosphorus spectra from healthy and cirrhotic liver tissue. The altered hepatic phosphorus metabolism in cirrhosis could be described by calculated molar concentrations of selected compounds in the liver tissue. The spectroscopic data of patients and controls together with Child-Pugh score are summarized in Table 1. Compared to controls, molar concentrations of PDE and ATP were significantly lower in all patients with liver cirrhosis. We divided patients into three groups according to CPS, independent of etiology. In this case, no significant differences were found between controls and patients with mild cirrhosis status (CPS-A) whereas groups CPS-B and CPS-C showed statistical differences to the control group in PDE (P<0.001) and ATP (P<0.02). If groups of patients were compared to each other, differences in patients within the CPS-A grouping increased for PDE as CPS worsened, no statistical differences were found in ATP. The relationship between calculated molar concentrations and the known etiology of liver cirrhosis is summarized in Table 2.
| n | PME | Pi | PDE | ATP | CPS | n | PME | Pi | |
| Controls | 11 | 3.09±1.45 | 1.63±0.55 | 10.83±2.68 | 3.72±0.99 | - | - | - | - |
| Alcohol | 33 | 3.48±1.53 | 1.19±0.39ac | 6.52±2.29df | 2.86±0.80b | A | 5 | 3.87±2.05 | |
| B | 6 | 3.50±1.32 | 1.17±0.35 | ||||||
| C | 22 | 3.38±1.51 | 1.21±0.43a | ||||||
| Viral | 22 | 3.64±1.55 | 1.57±0.77c | 6.47±3.13df | 2.84±0.92b | A | 7 | 3.47±1.92 | 1.54±0.69 |
| B | 8 | 3.99±1.51 | 1.51±0.63 | ||||||
| C | 7 | 3.40±1.41 | 1.66±1.04 | ||||||
| 1Cholestatic | 16 | 3.59±1.31 | 1.43±0.63 | 9.36±2.70 | 3.27±0.90 | A | 2 | - | - |
| B | 10 | 3.44±1.26 | 1.26±0.66 | ||||||
| C | 4 | - | - |
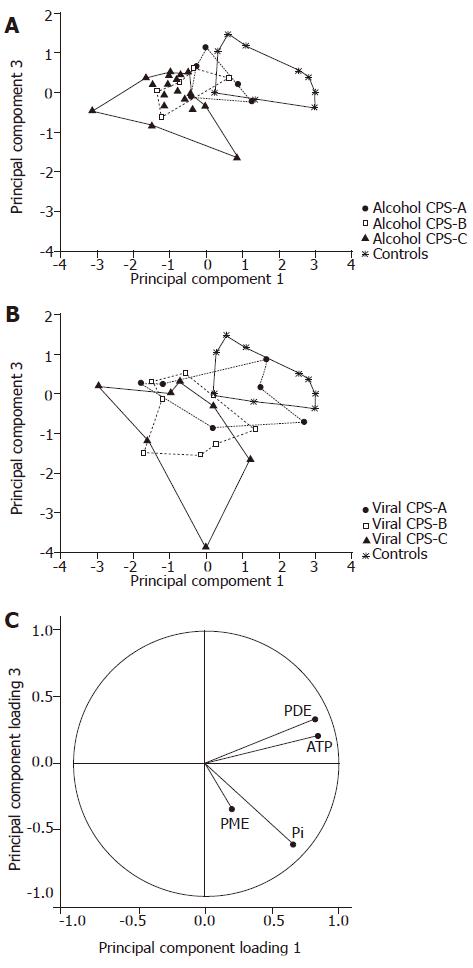
Spectroscopic data (PME, Pi, PDE and ATP concentrations) were also evaluated by various pattern recognition methods. We found similar levels of misclassification of subjects by using PCA, LDA and NDA procedures[15]. For demonstration and graphical output we used PCA plots from STATISTICA software[13], LDA and NDA results were not shown. To distinguish the different etiology and functional status of the liver cirrhosis from the controls, we highlighted individual subgroups of patients in PCA plots. If individual etiological groups were projected together with a control group, trends demonstrating the functional status of the liver were highlighted, i.e. the distance from the control area directly depended on CPS. The alcoholic group differed from controls in the CPS-C area, whereas the viral group differed in the CPS-B area (Figure 3). Because of the insufficient number of cholestatic patients in the CPS-A and CPS-C groups, that plot was not presented.
As data from the CPS-A groups overlapped with controls, we used patients with CPS-B or CPS-C liver status for another statistical analysis (Figure 4). In this case, principal component analysis confirmed clear separation of viral patients from controls. Partial overlap was seen between alcoholic patients and controls. Finally, the group of cholestatic patients overlapped all groups.
By comparing differences in 31P MR spectra of patients to controls we could distinguish alcoholic, viral and cholestatic etiologies of liver cirrhosis. Patients with alcoholic and viral etiology differed in PDE and ATP from the control group. Unlike viral etiology, patients with alcoholic etiology also differed from the control group in Pi (P<0.05). No significant changes were found in patients with cholestatic disease and the control group; however, this group differed from both alcoholic and viral groups in PDE.
Diagnosis of liver cirrhosis is mainly based on invasive methods such as liver biopsy, laparoscopy, various radiological examinations and other clinical tests. The functional severity of liver cirrhosis is usually described by CPS which is partially based on subjective parameters. Thus, this description is not fully sufficient and can impair accuracy. On the other hand, signals from 31P MR spectroscopy reflect intracellular and membrane metabolism in vivo non-invasively and they are objective parameters[6,7,8,10].
From our data above, we are able to conclude: (1) three basic etiologies can be distinguished by using correlation with metabolite concentrations; (2) there exists a correlation between the Child-Pugh score and the concentration of PDE and ATP, which is also observable in single etiologies.
The majority of published 31P MR spectroscopic studies have dealt with quantification of relative signal intensities characterized by ratios such as PCr/Pi, Pi/ATP etc. Unlike previous studies, where only relative signal ratios were used, we measured absolute concentration of the metabolites[11]. Absolute quantification of metabolites in mmol/L is not often used because of technical problems[12,16]. However, we believe that only relative quantification using signal intensity ratios cannot fully describe metabolic changes and absolute quantification should be taken into account even if a number of correction factors must be calculated. For example, if two or more compounds increase or decrease together, only the absolute quantification of independent metabolites accurately describes the event as signal ratios may remain unchanged.
PCA analysis of PME, Pi, PDE and ATP concentrations showed different localization of the etiological groups and trends in accordance with the CPS (Figures 3 and 4). Projection of the variables confirmed the similarities of ATP and PDE spectroscopic parameters. The best separation was obtained in the projection on the principal component-planes 1 and 3 despite losing some information available in the projections 1 and 2. The principal component 2 correlated specifically with PME. Nevertheless, in our study PME had high variation so that we did not use the projection on the component-plane 1 and 2.
Standard statistic tests confirmed the trends observable in PCA graphs. Data show a mean decrease of about 23% in ATP in patients with alcohol etiology versus the control group. A similar decrease of ATP concentration was also observed in the group of patients with viral etiology. In both groups we found that the decrease strongly depend on the CPS. Nevertheless, the higher difference was foud between CPS group A and CPS groups B and C. A very small difference was observed between CPS groups B and C which could be explained by the overall severity of hepatocyte dysfunction. Contrary to the alcoholic and viral groups, no statistically significant changes were found in ATP concentration in the cholestatic group.
Our findings in the group of alcoholic patients correspond to the results of animal studies. A number of reports show decreased levels of ATP in the liver of animals chronically fed ethanol. ATP levels could be measured using the intragastric feeding model[17,18]. In rats feeding a high-fat, low protein diet plus alcohol (similar to the diet of malnourished human alcoholics) levels of ATP decreased and remained constant at 35 % lower than levels of ATP in control animals. Hypoxia resulted in a decrease in hepatic levels of ATP in both ethanol-fed and control rats, but the magnitude of the decrease was significantly greater in the ethanol-fed group. Levels of adenosine monophosphate (AMP) and adenosine diphosphate (ADP) were not changed.
The most important change was a decreased concentration of PDE which is considered to be an indicator of membrane phospholipids and catabolic processes. The rate of PDE change was different in various etiologies. When etiological groups were compared to the control group regardless of CPS status (Table 2), mean PDE values were found to be about 60% in alcoholic and viral patients (P<0.001 to controls, and P<0.005 compared to the cholestatic group) and about 86% in the cholestatic group (without statistical significance). Moreover, in CPS-C groups, PDE concentration decreased to 39% of the control value in viral patients (58% in CPS-B) whereas in alcoholic patients PDE did not further decrease (57% of control PDE value in CPS-B and CPS-C). The fact that the changes are already significant in milder stages of cirrhosis (CPS-B) can improve the diagnostic effectiveness of this method. Unlike a previous study[9], our results were more pronounced in more severely affected patients.
We also studied PME concentration, which mainly represents intermediates on the phospholipid biosynthesis pathway. Although some other studies describe an increased PME signal[7,9,19-21], our data only show a statistically non-significant trend.
The last measured concentration - inorganic phosphate Pi - has been found to change only in alcoholic patients (P<0.05). Thus, inorganic phosphate concentration could be used to separate patients with alcoholic and viral etiology.
Observed signals in 31P MR spectra describe many metabolites in intra and extracellular liver tissue. It is known that many types of cells (hepatocytes, cholangiocytes, vascular wall cells, hepatic stellate cells macrophages and others) contribute to liver metabolism depending on their status. Changes in extracellular matrix also influence the concentration of energetic metabolites. Although 31P MR signals from the liver represent whole parenchymal tissue, the results of our study have confirmed previous findings that metabolic changes in energetic phospholipid metabolism are observable by 31P MRS in patients with decompensated cirrhosis in contrast to patients with compensated cirrhosis. In addition, different concentrations of metabolites in various etiologies indicate different damages of liver parenchyma.
In conclusion, according to differences in 31P MR spectra of patients and controls, we can differentiate various etiologies of liver cirrhosis, i.e. alcoholic, viral and cholestatic. Patients with alcoholic etiology differed in all selected metabolites except for PME from the control group. Patients with viral etiology differed from controls only in PDE and ATP, and no significant changes were found in patients with cholestatic disease. We suppose that this reflects different pathophysiological mechanisms of various liver diseases.
The importance of a larger, multicentric study to delineate ranges, borders and significant parameters for different cirrhotic groups (etiological and/or functional) in the nearest future is advisable. That will be the only way to assess the clinical relevance and usefulness of 31P MRS in liver cirrhosis. The application of this noninvasive method in liver patients will increase. The ultimate goal is the use of 31P MRS as a standard tool in the armamentarium of clinical hepatologists.
The authors thank Dr Karoly Heberger, Chemical Research Center, Hungarian Academy of Sciences, for the helpful discussion of the results.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Child CG, Turcotte JG. Surgery and portal hypertension. In child CG. The liver and portal hypertension. 1st ed. Philadelphia. WB Saunders Co. 1968;50-72. |
| 2. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5726] [Article Influence: 110.1] [Reference Citation Analysis (2)] |
| 3. | Christensen E, Schlichting P, Fauerholdt L, Gluud C, Andersen PK, Juhl E, Poulsen H, Tygstrup N. Prognostic value of Child-Turcotte criteria in medically treated cirrhosis. Hepatology. 1984;4:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 203] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Montgomery Bissel D, Maher JJ. Hepatic fibrosis and cirrhosis. Hepatology, a textbook of liver disease. 4th ed. Philadelphia: Saunders 2002; 395-416. |
| 5. | de Graff RA. In vivo NMR Spectroscopy: Principles and Techniques. 1st ed. Chichester: John Wiley & Sons 1998; . |
| 6. | Meyerhoff DJ, Boska MD, Thomas AM, Weiner MW. Alcoholic liver disease: quantitative image-guided P-31 MR spectroscopy. Radiology. 1989;173:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Munakata T, Griffiths RD, Martin PA, Jenkins SA, Shields R, Edwards RH. An in vivo 31P MRS study of patients with liver cirrhosis: progress towards a non-invasive assessment of disease severity. NMR Biomed. 1993;6:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Angus PW, Dixon RM, Rajagopalan B, Ryley NG, Simpson KJ, Peters TJ, Jewell DP, Radda GK. A study of patients with alcoholic liver disease by 31P nuclear magnetic resonance spectroscopy. Clin Sci (Lond). 1990;78:33-38. [PubMed] |
| 9. | Menon DK, Sargentoni J, Taylor-Robinson SD, Bell JD, Cox IJ, Bryant DJ, Coutts GA, Rolles K, Burroughs AK, Morgan MY. Effect of functional grade and etiology on in vivo hepatic phosphorus-31 magnetic resonance spectroscopy in cirrhosis: biochemical basis of spectral appearances. Hepatology. 1995;21:417-427. [PubMed] |
| 10. | Taylor-Robinson SD, Sargentoni J, Bell JD, Saeed N, Changani KK, Davidson BR, Rolles K, Burroughs AK, Hodgson HJ, Foster CS. In vivo and in vitro he patic 31P magnetic resonance spectroscopy and electron microscopy of the cirrhotic liver. Liver. 1997;17:198-209. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Tosner Z, Dezortová M, Tintĕra J, Hájek M. Application of two-dimensional CSI for absolute quantification of phosphorus metabolites in the human liver. MAGMA. 2001;13:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Murphy-Boesch J, Jiang H, Stoyanova R, Brown TR. Quantification of phosphorus metabolites from chemical shift imaging spectra with corrections for point spread effects and B1 inhomogeneity. Magn Reson Med. 1998;39:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Available from: http: //www.statsoft.com/textbook/stathome.html. |
| 14. | Meloun M, Militky J. Statisticka analyza experimentalnich dat. (Cz) (Statistical analysis of experimental data.) 1st ed. Academia. 2004;. |
| 15. | Available from: http: //www.eletel.P.lodz.pl/cost/cost_project.html. |
| 16. | Sijens PE, Dagnelie PC, Halfwerk S, van Dijk P, Wicklow K, Oudkerk M. Understanding the discrepancies between 31P MR spectroscopy assessed liver metabolite concentrations from different institutions. Magn Reson Imaging. 1998;16:205-211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Miyamoto K, French SW. Hepatic adenine nucleotide metabolism measured in vivo in rats fed ethanol and a high fat-low protein diet. Hepatology. 1988;8:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Corbin IR, Buist R, Peeling J, Zhang M, Uhanova J, Minuk GY. Hepatic 31P MRS in rat models of chronic liver disease: assessing the extent and progression of disease. Gut. 2003;52:1046-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Jalan R, Taylor-Robinson SD, Hodgson HJ. In vivo hepatic magnetic resonance spectroscopy: clinical or research tool? J Hepatol. 1996;25:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Kiyono K, Shibata A, Sone S, Watanabe T, Oguchi M, Shikama N, Ichijo T, Kiyosawa K, Sodeyama T. Relationship of 31P MR spectroscopy to the histopathological grading of chronic hepatitis and response to therapy. Acta Radiol. 1998;39:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Lim AK, Patel N, Hamilton G, Hajnal JV, Goldin RD, Taylor-Robinson SD. The relationship of in vivo 31P MR spectroscopy to histology in chronic hepatitis C. Hepatology. 2003;37:788-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |