Published online Nov 21, 2005. doi: 10.3748/wjg.v11.i43.6745
Revised: April 26, 2005
Accepted: April 30, 2005
Published online: November 21, 2005
AIM: To assess the sICAM-1, sVCAM-1, and sP-selectin levels in children with Helicobacter pylori (H pylori) infection and to evaluate their significance for the morphological changes found in gastric mucosa.
METHODS: The study included 106 children: 59 children (55.7%) with chronic gastritis and positive IgG against H pylori, 29 children (27.3%) after previous H pylori infection without the bacterium colonization but with positive IgG against H pylori, and 18 children (17%) with functional disorders of the gastrointestinal system but with normal IgG against H pylori. Endoscopic and histopathological evaluation of gastric mucosa was performed based on the Sydney System classification. The evaluation of sP-selectin, sICAM-1, sVCAM-1 levels in the sera of children was carried out using ELISA test.
RESULTS: The assessment of gastritis activity degrees indicated statistically significant values in the antrum and corpus (P<0.001) of children examined. Serum sVCAM-1 levels were higher in group with gastritis due to H pylori infection than in group without infection and differed statistically (P<0.05). Serum sVCAM-1 levels proved to be the highest among other adhesive molecules in infected children and decreased after eradication of H pylori. Serum sICAM-1 levels were similar in all examined groups. Serum sP-selectin levels were similar in children with and without H pylori infection.
CONCLUSION: Assessment of adhesive molecules (sP-selectin, sICAM-1, sVCAM-1) in the sera of children with active H pylori infection can show the participation of sVCAM-1 in the pathogenesis of gastric mucosal inflammation. sP-selectin and sICAM-1 concentrations in the sera of children with H pylori infection after eradication cannot reveal any significant differences as compared to healthy children.
-
Citation: Maciorkowska E, Kaczmarski M, Panasiuk A, Kondej-Muszynska K, Kemonai A. Soluble adhesion molecules ICAM-1, VCAM-1, P-selectin in children with
Helicobacter pylori infection. World J Gastroenterol 2005; 11(43): 6745-6750 - URL: https://www.wjgnet.com/1007-9327/full/v11/i43/6745.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i43.6745
In the course of Helicobacter pylori (H pylori) infection, a selective recruitment of neutrophils, monocytes, macrophages, mast cells, T and B lymphocytes takes place[1]. Additionally, infiltrating cells synthesize and release mediating cells, which influence the recruitment and activation of further inflammatory cells and increase the triggering activity of cytokines and chemokines. For example, neutrophils are the source of IL-1, IL-8, TNF-α[2], and macrophages – MIP-1α[1]. Adhesion molecules (selectins, their ligands, integrins, immunoglobulin-like molecules) take part in a selective recruitment of leukocytes[3-8]. Selectins are transmembrane molecules with numerous extracellular domains, containing lectin domain at the N-end – hence their name, L selectin (CD62L) – leukocyte, E selectin (CD62E) – endothelial cell, P-selectin (CD62P) – platelet. Saccharic residues combined with sialic acid [blood group antigen Lewis X (CD15) and its isoforms] expressing numerous leukocytes are ligands for L, E, and P selectins.
Glycosaminoglycans, such as heparin sulfate and carbohydrate residues on platelets and neutrophils, are ligands for L- and P-selectin[9,10]. A soluble form of P-selectin binds to the same ligand on granulocytes as its membrane form. It does not inhibit intergrins of granulocytes but supports agonists stimulating polymorphonuclear leukocytes, their function of secretion, PAF synthesis and LTB4 synthesis, and secretion. Thus, a soluble form does not differ in its activity from a membrane form. Its high serum levels (1-20 μg/mL) are significantly higher in the inflammatory process than in a healthy condition (36-250 ng/mL)[11].
Soluble forms of ICAM-1 (sICAM) were described in 1991 and are derived basically from mononuclear cells. Epithelial cells are unlikely to be their source[12]. They can be found in the serum, in molecular forms: 240 ku, 430 ku, and mainly 500 ku[13]. A functionally soluble ICAM-1 form can be regulated by cytokines and is able to bind to LFA-1 ligand. Thus, sICAM may compete with leukocytic ligands for binding and decrease leukocyte adhesion to endothelial cells and may even promote their de-adhesion. sICAM-1 may be regarded as a marker of inflammation, because its levels increase significantly in the serum in the course of inflammation, at tissue damage or during the activity of proteolytic enzymes[14]. In healthy individuals, ICAM-1 occurs in small amounts on surfaces of many cells, like leukocytes, endothelial vascular cells, fibroblasts, epithelial cells whereas in the course of inflammation correlates with chronic inflammatory phase as well as the occurrence of ulceration in the course of H pylori infection. ICAM-1 is considered to be a marker of chronic immunological stimulation and thus it is potentially responsible for chronic course of a disease. A vascular adhesion molecule (VCAM-1) requires about 8-96 h to be activated and expressed on cells in vitro. Its expression is enhanced especially by TNF-α, IL- 4, and IL-13[13]. Since VLA-4 is its ligand, it binds only to mononuclear leukocytes (lymphocytes and monocytes). Normal gastric mucosa is free of leukocytes. Abundant infiltrations of poly- and mono-nuclear cells and lymphatic follicles can be found in the course of H pylori infection.
The aim of the study was to evaluate the levels of adhesion molecules sICAM-1, sVCAM-1 and sP-selectin in the sera of children with H pylori infection and to determine their significance for morphological changes in gastric mucosa.
The study included 106 patients, who were divided into three groups with regard to the presence and course of H pylori infection. Group I : 59 children (55.7%) with chronic gastritis in the course of H pylori infection with a positive titer of IgG antibodies against H pylori, including 29 girls (49.2%) and 30 boys (50.8%). The children’s age ranged from 2 to 19 years, the mean age was 12.2±4.6 years.
Group II : 29 children (27.3%) after previous H pylori infection, without the bacterium colonization of the gastric mucosa but with a positive titer of IgG antibodies against H pylori, including 14 girls (14%) and 15 boys (51.7%). The children’s age ranged from 3 to 19 years and the mean age was 11.0±4.1 years.
Group III : 18 children (17 %) with functional disorders of the gastrointestinal tract, without H pylori infection but with normal IgG level against H pylori, 12 girls (66.7%) and 6 boys (33.3%). The children’s age ranged from 5 to 17 years, and the mean age was 10.7±3.6 years (Table 1).
| Groups | n(g/b)1 | Min.value | Max.value | Meanarithmetic | Median | SD | Lowerquartile | Upperquartile |
| Group I | 46 (30/29) | 2.0 | 19.0 | 12.2 | 13.0 | 4.6 | 9.0 | 16.0 |
| Group II | 17 (14/15) | 3.0 | 19.0 | 11.0 | 11.0 | 4.2 | 8.0 | 14.0 |
| Group III | 18 (12/6) | 5.0 | 17.0 | 10.7 | 10.0 | 3.6 | 8.0 | 13.0 |
Ethical approval for the research was obtained from local Ethics Committee in Medical University.
Endoscopic examination of the upper gastrointestinal tract with gastric mucosa samples (corpus and antrum) was performed in 88 children with positive IgG against H pylori and 18 children with negative IgG against H pylori. Endoscopic and histopathological evaluation was performed based on the Sydney System[16]. Chronic stomachache indicated the need for endoscopy. The assessment of sP-selectin (sP-selectin, Bender MedSystems, Austria), sICAM-1 (sICAM, Bender MedSystem) and sVCAM-1 (sVCAM, Bender MedSystems) levels in the serum samples was performed using ELISA method. The materials for the examination and assessment of individual parameters were prepared according to the manufacturer’s instructions. The results were read in a spectrophotometer at 450 nm wavelength. The minimum detection threshold in the method used equaled 1.3 ng/mL for sP-selectin, 0.5 ng/mL for sICAM-1, and 0.9 ng/mL for sVCAM-1.
The results of laboratory analysis were processed using appropriate calculating techniques and statistical tests. Descriptive statistics with central deviation measure including arithmetic mean (χ), median and mode, and measure of dispersion including standard deviation (SD), variations and values of the upper and lower quartiles were given to each variable (feature) measured and group of patients. The distribution of empirical data matching the normal distribution was checked using χ2 test and the Kolmogorow–Smirnow test. The results enabled to determine the direction of further statistical analysis and to use parametric tests or their non-parametric equivalents in statistical analysis. Since in most cases, formal normality tests proved that variations differed significantly from assumed theoretical distribution, the Mann–Whitney U test was used to examine the significance of difference in the feature intensity between the groups examined. P<0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves were applied to the estimation of the usability of individual diagnostic parameters.
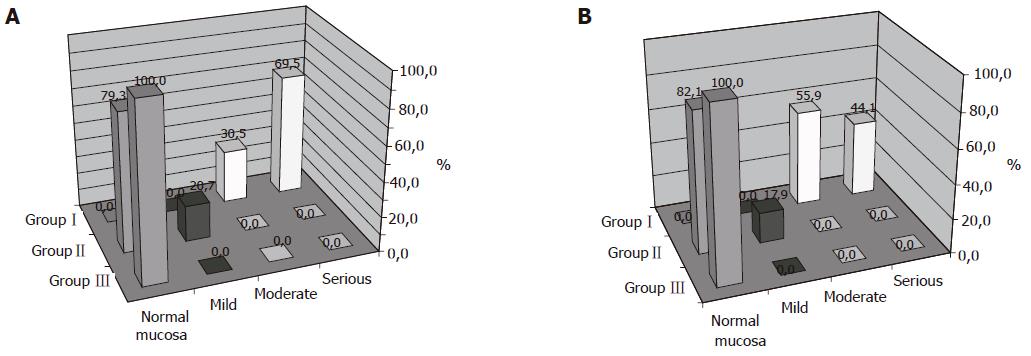
While evaluating the activity of antrum gastritis in groups, we showed the largest changes in children with H pylori infection (group I). The severe degree activity was found in 69.5% of this group and the moderate degree activity in 30.5% of the infected children. In children with previous infection and after bacteria eradication (group II), no severe or moderate degree activity was found, whereas mild degree activity was revealed only in 20.7% of this group (Figure 1A).
The analysis of antral gastritis activity by χ2 test proved a statistical significance (P<0.001) in examined groups. While the corpus mucosa in children with H pylori infection (group I) was assessed, the moderate degree activity was found in 55.9% of children and the severe degree activity was revealed in 44.1% of children. In children after H pylori eradication (group II), the mild degree activity was established only in 17.9%. No severe or moderate degree activity was reported (Figure 1B). The histopathological evaluation of the corpus gastritis differed statistically significant in particular groups (P<0.001).
sP-selectin levels equaled 339.2±122.9 ng/mL in the sera of children with H pylori infection (group I). The similar levels of sP-selectin were observed in children after H pylori eradication (group II) and in controls. Therefore, no statistically significant differences were found between the groups examined (Table 2). Based on the results of ROC analysis of sP-selectin levels in serum, the usefulness of this parameter was not confirmed (AUC = 0.62±0.08). The highest accuracy was obtained when the level of 1 288.2 ng/mL was taken as the criterion of sP-selectin concentration. The sensitivity was 87.2% and the specificity was 52.9% for this value (Figure 2A).
| Groups | n | Min. value | Max. value | Mean arithmetic | Median | Mode | SD | Lower quartile | Upper quartile |
| Group I | 46 | 158 | 612 | 339.2 | 312 | 343 | 122.9 | 248 | 380 |
| Group II | 17 | 127 | 624 | 389.1 | 375.2 | – | 151.5 | 283 | 509 |
| Group III | 3 | 215 | 330 | 283.7 | 306 | – | 60.7 | 261 | 318 |
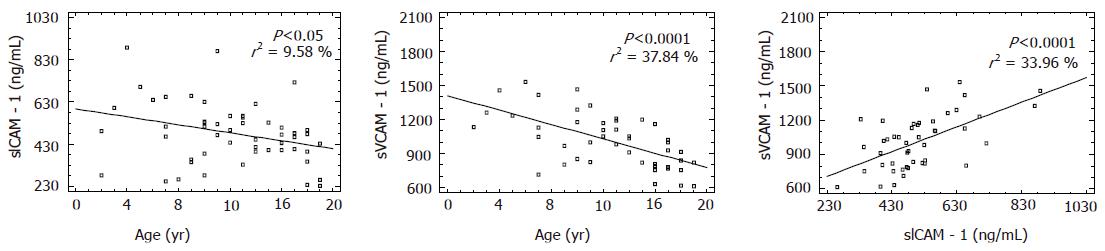
Soluble ICAM level was 482.3±143.2 ng/mL in children with H pylori infection (group I). Similar sICAM-1 levels were observed in children after H pylori eradication (group II) and in controls. No statistically significant differences of sICAM levels were proved between/among the groups (Table 3). A linear correlation was proved between the age of children with H pylori infection (group I) and sICAM levels. A negative value of a slope of a line (b = –9.43±3.98) (according to the Sydney System) indicates that sICAM levels decreased in the serum as the age increased. The value of the line equaled 597.18±51.91 ng/mL. This correlation was statistically significant (P<0.05) (Figure 3A).
| Groups | n | Min. value | Max. value | Mean arithmetic | Median | Mode | SD | Lower quartile | Upper quartile |
| Group I | 55 | 232 | 887 | 482.3 | 478 | 514 | 143.2 | 399 | 548 |
| Group II | 23 | 176 | 930 | 490.5 | 487.7 | – | 164.7 | 412 | 580 |
| Group III | 5 | 304 | 705 | 475.3 | 446 | – | 168.1 | 394 | 527 |
Soluble VCAM-1 levels in the sera of children with H pylori infection equaled 1 032.7±267.5 ng/mL. A statistically significant difference was proved between the levels of group I and group II (P<0.05) (Table 4). In children with H pylori infection, a statistically significant dependence (P<0.001) was revealed between the age of children examined and sVCAM-1 levels in their sera. This dependence can be described by a general formula: y = a+b×x. A negative value of a slope of a line (b = –31.67±–5.18) means that the value of sVCAM-1 levels decreased as the age of children increased (Figure 3B). Based on the results of ROC analysis of sVCAM levels in the serum, the usefulness of this parameter in H pylori infection and eradication was not confirmed (AUC = 0.69±0.08). In case of sVCAM-1 levels, the highest accuracy of diagnosis was obtained at the criterion of 1 288.2 ng/mL. The sensitivity was 87.2% and the specificity was 52.9% (Figure 2B).
| Groups | n | Min. value | Max.value | Mean arithmetic | Median | Mode | SD | Lower quartile | Upper quartile |
| Group I | 47 | 612 | 1 921 | 1 032.7 | 1 018.0 | 819 | 267.5 | 819 | 1 183.0 |
| Group II | 23 | 176 | 930 | 490.5 | 487.7 | – | 164.7 | 412 | 580 |
| Group III | 5 | 367 | 523 | 422.1 | 400.2 | – | 59.7 | 398.3 | 421.2 |
In children with H pylori infection, a linear dependence (y = a+b+x) was proved between sICAM-1 and sVCAM-1 levels in the serum. A positive value of a slope of a line (b = 1.08±0.23) indicates that sICAM-1 levels increased simultaneously with sVCAM-1 levels (Figure 3C).
The evaluation of soluble adhesion molecule levels in serum can confirm their presence and assess their levels quickly and therefore may be of better use in the diagnosis of H pylori infection or eradication than time-consuming immunohistochemical methods.
The levels of sVCAM-1 were higher in the sera of patients with gastritis of H pylori etiology than in patients without infection and differed significantly (P<0.05). The levels of sICAM in the sera were similar in all groups examined. The levels of sP-selectin were similar in the groups with or without H pylori infection but were twice as high as in controls. Similar results were presented in studies evaluating ICAM-1, VCAM-1 molecules on the surface of gastric epithelium[17-19].
Later studies in patients with chronic gastritis in the course of H pylori infection have proved that the predominant increase in ICAM-1 expression on vascular epithelial cells and inflammatory cells (lymphocytes, granulocytes) in lamina propria is connected with a massive inflammatory infiltrate and the expression of HLA-DR, LFA-1, and Mac-1 on cells presenting antigen[7,20]. No ICAM-1 expression was found on endothelial lymphocytes and epithelial cells. Similar to our study, no correlation with the degree of gastritis was proved. A decrease in the level of adhesion molecules examined was observed after effective eradication of H pylori.
Hatz et al[21] have proved that ICAM-1 expression increases on endothelial cells. Moreover, they observed an increase in VCAM - expression in lymphatic follicles, though they neither found an increase in P-selectin expression nor any E-selectin expression. According to the authors, constant P-selectin levels (no increase) may be due to a quick metabolism of this molecule (in vitro it is decomposed after a few minutes after its exposition on epithelial cells) and undetectable changes of its levels in immunohistopathological examination. As stated by Hatz et al[21], the increased levels of proinflammatory cytokines such as IL-1b and TNF-α and the increased quantity of CD4+ and CD45RO lymphocytes in lamina propria might contribute to the upregulated expression of ICAM-1 and VCAM-1. However, studies examining ICAM-1, VLA-4, and CD44 expressions on the surface of mononuclear cells in the serum have proved their increased quantity together with the expression of the molecules mentioned above in patients with H pylori infection but without ulceration and in healthy people[3,5]. Polymorphonuclear cells react similarly. The enhanced adhesion of these cells to the epithelial cells of the human navel vein exposed to H pylori antigens has been shown in laboratory tests evaluating ICAM-1, VCAM-1, E-selectin expression on neutrophils[6].
Innocenti et al[4] found that not all H pylori strains are able to activate epithelial cells of gastric mucosa to the expression of adhesion molecules (ICAM-1, VCAM-1, E-selectin) and chemokines for neutrophils. But the authors failed to prove whether combination of bacterial antigens (CagPaI, Lewis, BabA, VacA) could influence H pylori capability of activating epithelial cells. According to these authors, bacterial proteins not described so far take part in the activation of epithelial cells.
While the interdependence was evaluated between adhesion molecules, a strong correlation was found between the serum levels of sICAM and sVCAM-1 (P<0.001) in children with H pylori infection, indicating that the increase in sICAM-1 levels is accompanied with the increase in sVCAM-1 levels. Such a correlation may point to the simultaneous and proportional contribution of both adhesion molecules in inflammation.
In our study, while the correlation of sICAM-1 and sVCAM-1 levels with the age of children examined in groups I and II was evaluated, the highest levels were found in the youngest children, whereas they decreased gradually as the age of patients increased. The correlation was statistically significant in both groups (I and II) for sICAM (P<0.05), whereas for sVCAM in group I (P<0.0001) and in group II (P<0.05). The variability of sICAM-1 and sVCAM levels in serum regarding the age may suggest a greater maturity of children’s immunological system and its reaction to bacterial antigens.
The results of our study and other studies indicate that adhesion molecules play an important role in immuno-inflammatory response in patients with gastritis due to H pylori infection. The levels of adhesion molecules increase in inflammatory process. In our study, such a correlation was proved for sP-selectin.
The quantity of adhesion molecules in inflammatory infiltrating cells or the increased levels of their soluble forms in the serum correlate with the intensity of inflammatory process.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Kusugami K, Ando T, Imada A, Ina K, Ohsuga M, Shimizu T, Sakai T, Konagaya T, Kaneko H. Mucosal macrophage inflammatory protein-1alpha activity in Helicobacter pylori infection. J Gastroenterol Hepatol. 1999;14:20-26 DOI : 10.1046/j.1440-1746.1999.01810.x. |
| 2. | Kim JS, Jung HC, Kim JM, Song IS, Kim CY. Interleukin-8 expression by human neutrophils activated by Helicobacter pylori soluble proteins. Scand J Gastroenterol. 1998;33:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Ohara T, Arakawa T, Higuchi K, Kaneda K. Overexpression of co-stimulatory molecules in peripheral mononuclear cells of Helicobacter pylori-positive peptic ulcer patients: possible difference in host responsiveness compared with non-ulcer patients. Eur J Gastroenterol Hepatol. 2001;13:11-18 DOI : 10.1097/00042737-200101000-00003. |
| 4. | Innocenti M, Thoreson AC, Ferrero RL, Strömberg E, Bölin I, Eriksson L, Svennerholm AM, Quiding-Järbrink M. Helicobacter pylori-induced activation of human endothelial cells. Infect Immun. 2002;70:4581-4590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Fan XG, Fan XJ, Xia HX, Keeling PW, Kelleher D. Up-regulation of CD44 and ICAM-1 expression on gastric epithelial cells by H. pylori. APMIS. 1995;103:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Byrne MF, Corcoran PA, Atherton JC, Sheehan KM, Murray FE, Fitzgerald DJ, Murphy JF. Stimulation of adhesion molecule expression by Helicobacter pylori and increased neutrophil adhesion to human umbilical vein endothelial cells. FEBS Lett. 2002;532:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Dobretsov GE, Kharitonenkov IG, Mishiev VE, Vladimirov IuA. Relation between fluorescence and circular dichroism of the complex of the fluorescence probe 4-dimethylaminochalcone with serum albumin. Biofizika. 1975;20:215-221. |
| 8. | Hatz RA, Rieder G, Stolte M, Bayerdörffer E, Meimarakis G, Schildberg FW, Enders G. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology. 1997;112:1908-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 753] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 10. | Moore KL. Structure and function of P-selectin glycoprotein ligand-1. Leuk Lymphoma. 1998;29:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Lorant DE, Topham MK, Whatley RE, McEver RP, McIntyre TM, Prescott SM, Zimmerman GA. Inflammatory roles of P-selectin. J Clin Invest. 1993;92:559-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 246] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987;51:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 1241] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 13. | Seth R, Raymond FD, Makgoba MW. Circulating ICAM-1 isoforms: diagnostic prospects for inflammatory and immune disorders. Lancet. 1991;338:83-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 284] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. A form of circulating ICAM-1 in human serum. J Immunol. 1991;147:3788-3793. [PubMed] |
| 15. | Kotowicz K, Dixon GL, Klein NJ, Peters MJ, Callard RE. Biological function of CD40 on human endothelial cells: costimulation with CD40 ligand and interleukin-4 selectively induces expression of vascular cell adhesion molecule-1 and P-selectin resulting in preferential adhesion of lymphocytes. Immunology. 2000;100:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3555] [Article Influence: 122.6] [Reference Citation Analysis (3)] |
| 17. | Enders G, Brooks W, von Jan N, Lehn N, Bayerdörffer E, Hatz R. Expression of adhesion molecules on human granulocytes after stimulation with Helicobacter pylori membrane proteins: comparison with membrane proteins from other bacteria. Infect Immun. 1995;63:2473-2477. [PubMed] |
| 18. | Scheynius A, Engstrand L. Gastric epithelial cells in Helicobacter pylori-associated gastritis express HLA-DR but not ICAM-1. Scand J Immunol. 1991;33:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Fan X, Long A, Fan X, Keeling PW, Kelleher D. Adhesion molecule expression on gastric intra-epithelial lymphocytes of patients with Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1995;7:541-546. [PubMed] |
| 20. | Archimandritis A, Sougioultzis S, Foukas PG, Tzivras M, Davaris P, Moutsopoulos HM. Expression of HLA-DR, costimulatory molecules B7-1, B7-2, intercellular adhesion molecule-1 (ICAM-1) and Fas ligand (FasL) on gastric epithelial cells in Helicobacter pylori gastritis; influence of H. pylori eradication. Clin Exp Immunol. 2000;119:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Hatz RA, Meimarakis G, Bayerdorffer E, Stolte M, Kirchner T, Enders G. Characterization of lymphocytic infiltrates in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1996;31:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |