Published online Nov 7, 2005. doi: 10.3748/wjg.v11.i41.6543
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: November 7, 2005
AIM: To study the pathogenetic processes and the role of gene expression by microarray analyses in expediting our understanding of the molecular pathophysiology of pancreatic adenocarcinoma, and to identify the novel cancer-associated genes.
METHODS: Nine histologically defined pancreatic head adenocarcinoma specimens associated with clinical data were studied. Total RNA and mRNA were isolated and labeled by reverse transcription reaction with Cy5 and Cy3 for cDNA probe. The cDNA microarrays that represent a set of 4 096 human genes were hybridized with labeled cDNA probe and screened for molecular profiling analyses.
RESULTS: Using this methodology, 184 genes were screened out for differences in gene expression level after nine couples of hybridizations. Of the 184 genes, 87 were upregulated and 97 downregulated, including 11 novel human genes. In pancreatic adenocarcinoma tissue, several invasion and metastasis related genes showed their high expression levels, suggesting that poor prognosis of pancreatic adenocarcinoma might have a solid molecular biological basis.
CONCLUSION: The application of cDNA microarray technique for analysis of gene expression patterns is a powerful strategy to identify novel cancer-associated genes, and to rapidly explore their role in clinical pancreatic adenocarcinoma. Microarray profiles provide us new insights into the carcinogenesis and invasive process of pancreatic adenocarcinoma. Our results suggest that a highly organized and structured process of tumor invasion exists in the pancreas.
- Citation: Jin G, Hu XG, Ying K, Tang Y, Liu R, Zhang YJ, Jing ZP, Xie Y, Mao YM. Discovery and analysis of pancreatic adenocarcinoma genes using cDNA microarrays. World J Gastroenterol 2005; 11(41): 6543-6548
- URL: https://www.wjgnet.com/1007-9327/full/v11/i41/6543.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i41.6543
Pancreatic carcinoma is the 12th most common cause of cancer death in China, which is one of the most aggressive form of human tumors and is virtually incurable. Its incidence and mortality rates are almost identical, even after receiving surgical resection and adjuvant chemo-radiotherapy, the overall 5-year survival rate is only 4.1%[1]. The etiology of pancreatic carcinoma is still unknown. There is clearly a need for novel and more effective diagnostic and therapeutic methods.
Oncogenesis and development of neoplasm is a complex multiphase process, which involves overexpression of oncogenes or inactivation of tumor suppressor genes, mutation or depletion of normal genes, pleiotropic effects and immunologic function. The genes are involved in vital processes of life, such as gene expression accommodation, immunology or cell differentiation, which are arranged in some gene clusters, in which all the members are being controlled in unity[2]. In the process of oncogenesis, there may exist some different clusters of tumor-related genes. Hence, it is important to find such unique gene clusters involved in carcinogenesis, invasion and metastasis processing.
Current focus of molecular profiling is the large-scale analysis of gene expression using new DNA array technology[3]. This powerful technology is being used to study many biological processes. The experimental or clinical goals range from insights to pathogenesis, cancer diagnosis and prediction of clinical outcome for identification of therapeutic targets. In this way, DNA array analysis is providing the first glimpse of a substantial improvement in our understanding of cancer biology and diagnosis. Identifying and sequencing a set of full-length cDNAs that represent all human genes would help in both gene discovery and functional analysis. It offers a great opportunity to study the pathogenetic processes and molecular pathophysiology of pancreatic carcinoma.
In this study, we analyzed nine pancreatic adenocarcinomas using the cDNA microarray containing 4 096 human genes with the aim of understanding expression patterns and searching for carcinogenesis-related gene clusters and novel useful markers for the malignant potential of pancreatic carcinoma at the molecular level.
We analyzed samples of pancreatic head adenocarcinoma from nine patients (five males, four females, 51-71 years) who underwent pancreaticoduodenectomy at Changhai Hospital, Shanghai, China, between November 1999 and May 2000. All samples were collected with informed consent and Ethics Committee approval. Samples were grossly dissected and snap-frozen in liquid nitrogen within 10 min of removal and stored at -80 °C. Initial diagnosis of each sample from the frozen section was later confirmed by detailed analysis of paraffin-embedded sections. Following the fourth Japanese edition of the Classification of Pancreatic Carcinoma (Japan Pancreas Society, 1993), the nine tumors were staged, including two stage I, two stage II, four stage III and one stage IVa. We isolated and purified total RNA from pooled, noncancerous, male adult human pancreas tissues and used as a reference “normal” sample for each microarray experiment.
Tumor and normal tissue samples were ground into a fine powder in a 10-cm ceramic mortar (RNase-free) and total RNA was extracted according to the original single-step extraction procedure with slight modifications. Ground tissue was homogenized in Solution D containing 1% ß-mercaptoethanol. After centrifugation, the supernatant was extracted twice with an equal volume of phenol:chloroform (1:1) and once with an equal volume of acidic phenol:chloroform (5:1), discarding the organic phase each time. The aqueous phase was then precipitated by an equal volume of isopropylalcohol at 4 °C, centrifuged to pellet the RNA and dissolved in deionized (Milli-Q) H2O. mRNA was purified using an Oligotex-dT mRNA Midi Kit (Qiagen, Inc., Carlsbad, CA, USA), following the manufacturer’s instructions. The RNA concentration was measured by using spectrophotometry, and its integrity was assessed by electrophoresis on formaldehyde-agarosegel.
The construction of the microarrays was carried out following Stanford University’s method[4]. The microarrays consist of 4 096 sequences, including full-length and partial complementary DNAs (cDNAs) representing novel, known and control genes provided by United Gene Holdings, Ltd. The known genes were selected from NCBI UniGene set and cloned into a plasmid vector. The novel genes were obtained through systematic full-length cloning efforts carried out at United Gene Holding, Ltd. The control spots of non-human origin in 4 096 chip included the rice U2 RNA gene (8 spots), hepatitis C virus (HCV) coat protein gene (8 spots), and spotting solution alone without DNA (32 spots). The cDNA inserts were amplified by PCR using universal primers and then purified. All PCR products were examined by electrophoresis on an agarose gel to ensure the quality and identity of amplified clones as expected. Then the amplified PCR products were dissolved in a buffer containing 3× SSC solution. The solutions with amplified PCR products were spotted onto silylated slides (CEL Associates, Houston, TX, USA) using a Cartesian PixSys 7 500 motion control robot (Cartesian Technologies, Irvine, CA, USA) fitted with ChipMaker Micro-Spotting Technology (TeleChem International, Sunnyvale, CA, USA). Glass slides with spotted cDNA were then hydrated for 2 h in 70% humidity, dried for 0.5 h at room temperature, and UV cross-linked at a dose of 65 mJ/cm. They were further treated with 2 g/L sodium dodecyl sulfate (SDS) for 10 min, distilled H2O for 10 min, and 2 g/L sodium borohydride (NaBH4) for 10 min at room temperature. The slides were dried again and made ready for use.
The fluorescent cDNA probes were prepared through reverse transcription of the isolated mRNAs and then purified. The RNA samples from healthy individuals were labeled with Cy3-dUTP and those from cancerous patients with Cy5-dUTP. The two color probes were then mixed, precipitated with ethanol and dissolved in 20 µL of hybridization solution. Microarrays were pre-hybridized with hybridization solution containing 0.5 mg/mL denatured salmon sperm DNA at 42 °C for 6 h. Fluorescent probe mixtures were denatured at 95 °C for 5 min, and the denatured probe mixtures were applied onto the pre-hybridized chip under a cover glass. Chips were hybridized at 42 °C for 15-17 h. The hybridized chips were then washed at 60 °C for 10 min each in solutions of 2× SSC and 2 g/L SDS, 0.1× SSC and 2 g/L SDS, and 0.1× SSC, and then dried at room temperature.
The chips were scanned with a ScanArray 3000 (GSI Lumonics, Billerica, MA, USA) at two wavelengths to detect emission from both Cy3 and Cy5. The acquired images were analyzed using ImaGene 3.0 software (BioDiscovery, Inc., Los Angeles, CA, USA). The intensities of each spot at the two wavelengths represent the quantity of Cy3-dUTP and Cy5-dUTP, respectively, hybridized to each spot. Ratios of Cy3-Cy5 were computed for each location on each microarray. Overall intensities were normalized with a correction coefficient obtained using the ratios of 40 housekeeping genes (available at http://www.biodoor.com/).
We used the threshold value to define significant relative expression changes, which set at 2.0 for overexpression and at 0.50 for underexpression on the basis of both the experimental variability in our data and the manufacturer’s established performance criteria. Data filtering with this algorithm identified the genes overexpressed at least by twofold and underexpressed at least by 50% across, more than 66.7% (6/9) of all specimens. To minimize artifacts arising from low expression values, only the genes with raw intensity values for both Cy3 and Cy5 of >600 counts were chosen for differential analysis.
The purity and concentration of isolated RNA were analyzed first by using UV spectrophotometer at absorbance wavelengths of 260 and 280 nm (A260 and A280). The average A260/A280 ratio was higher than 1.9. Furthermore, the integrity of the RNA sample was verified by electrophoresis on 10 g/L agarose gel stained with ethidium bromide. The quality of the RNA was assessed by the visualization of the 28S and 18S ribosomal RNA bands. The bands were distinct and sharp, without being diffused and smeared. The results indicated that mRNA preparation expressed continuous polyadenylated transcripts between 0.9 and 4.0 kb in length.
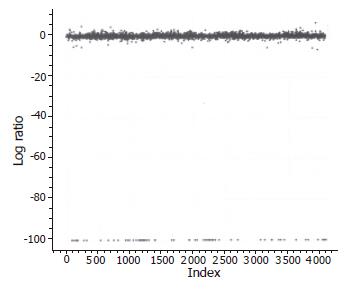
In order to access the “noise” in the differential expression assay, we employed self-comparison experiments. A sample of mRNA from a single fetal liver tissue was divided into two equal aliquots and labeled with Cy3-dUTP and Cy5-dUTP, respectively. The labeled samples were then mixed together and hybridized to the microarray. The results revealed that approximately 1% of the 4 096 cDNA clones showed more than 2.0-fold difference in signal intensity between the two channels. Furthermore, this “noise” in the data was shown upon analysis to occur at random array positions in each microarray experiment. Figure 1 shows the scatter plots of the within-slide normalization experiment. The Cy3/Cy5 log ratios from the different print tip groups were centered around zero, indicating that the types of systematic errors were minimized. The spots in the experiments showed a highly concordant distribution pattern. Besides, hybridization experiments with probes prepared from human mRNA produced little or no signal at the positions of the negative controls (data not shown).
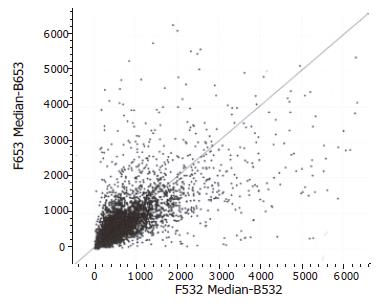
The hybridizations for each individual were repeated twice. Of all the 4 096 human genes analyzed through microarray experiments, a total of 184 genes (4.5%) revealed differential expression in more than 60.66% (6/9) of the pancreatic adenocarcinoma specimens using a fold ratio of >2 as criteria for cut-off. Of 184 genes, the expression of 87 genes (47.3%) was markedly increased in pancreatic adenocarcinoma tissues as compared with the normal pancreas tissues (Figure 1). In addition, the expression of 97 genes (52.7%) was significantly decreased in pancreatic adenocarcinoma tissues as compared with normal pancreatic tissues (Figure 2). The 184 genes corresponded to 173 genes represented in the GenBank and 11 novel sequences which have not been found in the GenBank. Scatter plots with cancerous tissues showed a wide distribution pattern (Figure 2), suggesting that genes are expressed differentially in cancer cells when compared with the normal cells. Scatter plot of the values of Cy3 and Cy5 fluorescent signals also revealed a pattern of tight distribution and clustered in an almost 45° diagonal line as expected.
In the 11 novel genes screened by our microarray experiments, an overexpressed clone in pancreatic adenocarcinomas was identified. The average Cy5/Cy3 ratio of the clone is 4.92. As we have reported recently[5], this clone is the full-length cDNA of the human gene S100P (GenBank accession number AF539739). The sequence is of 1 297 bp and encodes a protein identical to previously characterized human S100P, but it is much longer than the previously reported 439 bp. The cDNA is near full-length as confirmed by Northern blot analysis. We examined its distribution in tissues by using Northern blot and RT-PCR analysis, and found that it was abundantly expressed in many tissues including placenta, unlike the expression pattern of other S100 family genes.
DNA microarray technology has offered us a new insight into the secrets of life by monitoring the activities and profiles of thousands clones simultaneously. The gene expression profiles can led us to mapping a cross-section of genetic activities and biological entity[6].
In this experiment, the genes identified as differentially expressed in microarrays revealed a wealth of information that pancreatic adenocarcinomas are complex tumors, as evidenced by the wide range of investigations[7-9]. However, these findings not only provide novel insight into the biology of pancreatic carcinoma, but also serve to identify numerous new targets for development into serologic markers or therapeutic target. The differentially expressed genes in pancreatic adenocarcinomas included oncogenes and tumor suppression genes, cell-cycle-related genes, signal transduction factors, extracellular matrix and skeleton related genes, transcription factors, DNA damage and repair related genes and apoptosis-related genes (Tables1 and 2).
The screened genes with good concordance in these pancreatic adenocarcinoma patients may have the potential to become candidates for tumor markers and the molecular target for gene therapy, whereas genes that show concordance in a patient subset may reflect different disease stages or physiological and genetic differences between the patients.
Griffin et al[10] reported that more than 70% of pancreatic adenocarcinomas possessed consistent chromosome abnormalities. The most frequent whole chromosomal gains were chromosomes 20 and 7, and the chromosomal losses were much more frequent in chromosomes 18, 13, 12, 17, and 6. Structural abnormalities were frequently involved in chromosomes 1p, 6q, 7q, 17p, 1q, 3p, 11p, and 19q. From our microarrays, we found that the overexpressed genes in pancreatic adenocarcinomas are mainly located in chromosomes 1, 2q, 7q, 9q, 12, 14q, 15q, and 21q, and the downexpressed genes are mainly located in chromosomes 1, 2, 3, 7q, 8q, 15q, 17, 19, and 22q, which are similar to the previous reports. This phenomenon suggests the existence of acquired genomic alterations in pancreatic carcinomas.
Among the genes overexpressed in pancreatic adenocarcinomas, RAB22B and Rho GDP dissociation inhibitor (Rho GDI) are the members of Ras superfamily, whose Cy3/Cy3 ratios are 3.53 and 4.504, respectively. As it is well known that many pancreatic carcinoma cells show “addiction” to K-ras mutation, while normal cells appear resistant to suppression of K-ras-mediated signaling by antisense K-ras RNA expression adenoviral vector[11]. So, overexpressed RAB22B may be the result of K-ras mutation in pancreatic adenocarcinoma. The Rho family proteins were found to reorganize cytoskeletons and regulate the cell migration via the activation of effector proteins. GTP-bound Rho is an active form, whereas the GDP-bound form is inactive. Rho GDI can block the conversion between the GTP- and GDP-bound forms. Expression of Rho family molecules has recently been reported in breast, lung, pancreas and colon carcinomas, and testicular germ cell tumors[12]. Moreover, three guanine nucleotide exchange factors (RCC1-like G exchanging factor, eukaryotic translation elongation factor 1-δ, eukaryotic translation elongation factor 1-β2) were downexpressed in our microarrays, which implied that GDP-bound forms might be related to tumorigenesis of pancreatic adenocarcinoma. Furthermore, VHL gene, which has been confirmed as a tumor suppressor gene[13], was also downexpressed in pancreatic adenocarcinoma.
The result showed that pancreatic carcinoma cells are much more active than normal cells in many steps of multiple pathways of signal transduction. The stable state of normal somatic cells depends on the dynamic equilibrium of apoptosis and proliferation. Apoptosis-related genes were downexpressed in cancer. These findings revealed that the phenotypical similarities among different cancers are also reflected at the molecular level.
Gene expression profiling of pancreatic carcinoma has also provided new insights into the process of tumor invasion. In pancreatic carcinoma tissue, many invasion and metastasis related genes, such as ECM and cell skeleton related genes (type I collagen, type III collagen, type IV collagen, decorin, secreted phosphoprotein 1, vimentin, tissue inhibitor of matrix metalloproteinase 1, fibronectin 1,α2-actin, tubulin, tropomyosin 1, etc.), showed high expression level, reflecting the cellular components of the host stromal response seen in the presence of infiltrating carcinoma. Moreover, the urokinase-type plasminogen activator receptor (uPAR) was found highly expressed in pancreatic adenocarcinomas, which is a key molecule in the regulation of cell-surface plasminogen activation and, as such, plays an important role in many normal as well as pathologic processes[14]. Memarzadeh et al[15] concluded that uPAR is a useful prognostic marker for biologically aggressive forms of endometrial cancer. These phenomena suggest that poor prognosis of pancreatic carcinoma may have a solid molecular biological basis, and also indicate that a highly organized and structured process of tumor invasion exists in the pancreas.
The downregulated genes in the patients with pancreatic adenocarcinoma are also divided into distinct functional categories. Reduced expression was observed in genes encoding products that function in the apoptosis, immune system, cell regulation, DNA injury and repair processing and GTP/GDP signaling, which were in agreement with the previous reports[16,17].
In conclusion, the application of cDNA microarray technique for analysis of gene expression patterns is a powerful strategy to identify novel cancer-associated genes, and can rapidly explore their role in clinical pancreatic adenocarcinomas. Microarray profiles provide us new insights into the carcinogenesis and invasive process in pancreatic adenocarcinoma. Our results suggest that a highly organized and structured process of tumor invasion exists in the pancreas.
Science Editor Kumar M, Li WZ and Guo SY Language Editor Elsevier HK
| 1. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-257; discussion 257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1387] [Article Influence: 49.5] [Reference Citation Analysis (34)] |
| 2. | Holland EC. Regulation of translation and cancer. Cell Cycle. 2004;3:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Wulfkuhle J, Espina V, Liotta L, Petricoin E. Genomic and proteomic technologies for individualisation and improvement of cancer treatment. Eur J Cancer. 2004;40:2623-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Shalon D, Smith SJ, Brown PO. A DNA microarray system for analyzing complex DNA samples using two-color fluorescent probe hybridization. Genome Res. 1996;6:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 588] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 5. | Jin G, Wang S, Hu X, Jing Z, Chen J, Ying K, Xie Y, Mao Y. Characterization of the tissue-specific expression of the s100P gene which encodes an EF-hand Ca2+-binding protein. Mol Biol Rep. 2003;30:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4800] [Cited by in RCA: 4423] [Article Influence: 192.3] [Reference Citation Analysis (0)] |
| 7. | Missiaglia E, Blaveri E, Terris B, Wang YH, Costello E, Neoptolemos JP, Crnogorac-Jurcevic T, Lemoine NR. Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int J Cancer. 2004;112:100-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Ryu B, Jones J, Blades NJ, Parmigiani G, Hollingsworth MA, Hruban RH, Kern SE. Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res. 2002;62:819-826. [PubMed] |
| 9. | Tarbé N, Lösch S, Burtscher H, Jarsch M, Weidle UH. Identification of rat pancreatic carcinoma genes associated with lymphogenous metastasis. Anticancer Res. 2002;22:2015-2027. [PubMed] |
| 10. | Griffin CA, Hruban RH, Morsberger LA, Ellingham T, Long PP, Jaffee EM, Hauda KM, Bohlander SK, Yeo CJ. Consistent chromosome abnormalities in adenocarcinoma of the pancreas. Cancer Res. 1995;55:2394-2399. [PubMed] |
| 11. | Yoshida T, Ohnami S, Aoki K. Development of gene therapy to target pancreatic cancer. Cancer Sci. 2004;95:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Aznar S, Fernández-Valerón P, Espina C, Lacal JC. Rho GTPases: potential candidates for anticancer therapy. Cancer Lett. 2004;206:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Maynard MA, Ohh M. Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer. Am J Nephrol. 2004;24:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Choong PF, Nadesapillai AP. Urokinase plasminogen activator system: a multifunctional role in tumor progression and metastasis. Clin Orthop Relat Res. 2003;S46-S58. [PubMed] |
| 15. | Memarzadeh S, Kozak KR, Chang L, Natarajan S, Shintaku P, Reddy ST, Farias-Eisner R. Urokinase plasminogen activator receptor: Prognostic biomarker for endometrial cancer. Proc Natl Acad Sci USA. 2002;99:10647-10652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Grützmann R, Foerder M, Alldinger I, Staub E, Brümmendorf T, Röpcke S, Li X, Kristiansen G, Jesenofsky R, Sipos B. Gene expression profiles of microdissected pancreatic ductal adenocarcinoma. Virchows Arch. 2003;443:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R, Hanash S. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649-2657. |