Published online Nov 7, 2005. doi: 10.3748/wjg.v11.i41.6450
Revised: May 21, 2005
Accepted: May 24, 2005
Published online: November 7, 2005
AIM: Grapefruit-seed extract (GSE) containing flavonoids, possesses antibacterial and antioxidative properties but whether it influences the gastric defense mechanism and gastroprotection against ethanol- and stress-induced gastric lesions remains unknown.
METHODS: We compared the effects of GSE on gastric mucosal lesions induced in rats by topical application of 100% ethanol or 3.5 h of water immersion and restraint stress (WRS) with or without (A) inhibition of cyclooxygenase (COX)-1 activity by indomethacin and rofecoxib, the selective COX-2 inhibitor, (B) suppression of NO-synthase with L-NNA (20 mg/kg ip), and (C) inactivation by capsaicin (125 mg/kg sc) of sensory nerves with or without intragastric (ig) pretreatment with GSE applied 30 min prior to ethanol or WRS. One hour after ethanol and 3.5 h after the end of WRS, the number and area of gastric lesions were measured by planimetry, the gastric blood flow (GBF) was assessed by H2-gas clearance technique and plasma gastrin levels and the gastric mucosal generation of PGE2, superoxide dismutase (SOD) activity and malonyldialdehyde (MDA) concentration, as an index of lipid peroxidation were determined.
RESULTS: Ethanol and WRS caused gastric lesions accompanied by the significant fall in the GBF and SOD activity and the rise in the mucosal MDA content. Pretreatment with GSE (8-64 mg/kg i g) dose-dependently attenuated gastric lesions induced by 100% ethanol and WRS; the dose reducing these lesions by 50% (ID50) was 25 and 36 mg/kg, respectively, and this protective effect was similar to that obtained with methyl PGE2 analog (5 μg/kg i g). GSE significantly raised the GBF, mucosal generation of PGE2, SOD activity and plasma gastrin levels while attenuating MDA content. Inhibition of PGE2 generation with indomethacin or rofecoxib and suppression of NO synthase by L-NNA or capsaicin denervation reversed the GSE-induced protection and the accompanying hyperemia. Co-treatment of exogenous calcitonine gene-related peptide (CGRP) with GSE restored the protection and accompanying hyperemic effects of GSE in rats with capsaicin denervation.
CONCLUSION: GSE exerts a potent gastroprotective activity against ethanol and WRS-induced gastric lesions via an increase in endogenous PG generation, suppression of lipid peroxidation and hyperemia possibly mediated by NO and CGRP released from sensory nerves.
-
Citation: Brzozowski T, Konturek PC, Drozdowicz D, Konturek SJ, Zayachivska O, Pajdo R, Kwiecien S, Pawlik WW, Hahn EG. Grapefruit-seed extract attenuates ethanol-and stress-induced gastric lesions
via activation of prostaglandin, nitric oxide and sensory nerve pathways. World J Gastroenterol 2005; 11(41): 6450-6458 - URL: https://www.wjgnet.com/1007-9327/full/v11/i41/6450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i41.6450
Grapefruit-seed extract (GSE) containing flavonoids, possesses antibacterial, antiviral and antifungal properties[1-3]. These beneficial actions of GSE have been attributed to the antioxidative activity of grapefruit containing citrous flavonoids such as naringenin[4]. Moreover, grapefruit juice and its major flavonoid exhibited the potent anti-H pylori activity in vitro[5] and was also recently found to exert the cytoprotection against hepatocyte injury induced by algal toxins[6]. In another study, naringenin showed anti-cancer activity against human breast cancers[7]. Therapeutic efficacy of citrous fruits such as grapefruits and red grapes has been explained by the content of different classes of polyphenolic flavonoids, that were shown to inhibit platelet aggregation, thus decreasing the risk of coronary thrombosis and myocardial infarction[8]. However, the involvement of grapefruit extracts containing various flavonoids in the mechanism of gastric mucosal defense has been little studied.
Our group demonstrated previously that other flavonoids, namely, meciadanol, a synthetic flavonoid inhibiting histidine decarboxylase (HDC) and decreasing histamine content in the stomach, attenuated gastric mucosal lesions produced by ethanol and aspirin via the mechanism unrelated to gastric acid secretion and endogenous prostaglandins (PG)[9]. Furthermore, naringenin, a major citrous flavonoid, was reported to exhibit gastroprotection against the gastric injury induced by absolute ethanol due to the increase in the mucus secretion and that this gastroprotective effect of naringenin and accompanying increase in the mucus secretion, were, in part, attenuated by indomethacin suggesting the involvement of endogenous PG in the mechanism of this flavonoid-induced gastroprotection[10]. It remains unknown, to what extent GSE influences the gastric mucosal injury induced by topical (ethanol) and non-topical ulcerogens (stress) and, if so, what is the mechanism of gastroprotection induced by GSE. Therefore, using animal models of gastric lesions induced by 100% ethanol and water immersion and restraint stress (WRS), we determined the influence of GSE on gastric lesions and the accompanying changes in the gastric blood flow (GBF) in the rat stomach. An attempt was also made to assess the contribution of gastric acid secretion, plasma gastrin levels, PG/cyclooxygenase (COX)-system, nitric oxide (NO) and sensory nerves in the gastroprotective effect of GSE. Finally, we evaluated the effect of pretreatment with GSE on the gene expression and activity of superoxide dismutase (SOD) and lipid peroxidation, as expressed by malonyldialdehyde (MDA) concentration, in rats with or without the pretreatment with GSE.
Male Wistar rats, weighing 180-220 g and fasted for 24 h before the study were employed in all the studies. This study was approved by the Institutional Animal Care and Use Committee of Jagellonian University School of Medicine in Cracow and run in accordance to the statements of European Union regarding handling of experimental animals.
The effects of GSE purchased from Herb-Pharma, Welke Dudince, Slovakia on gastric acid secretion were examined in 40 conscious rats equipped about 1 mo earlier with a gastric fistula (GF) as described previously[11]. The animals were fasted overnight but had free access to water 24 h before the experiment and they were placed in individual Bollman type cages to maintain the minimum restraint necessary. The GF was opened; the stomach was rinsed gently with about 5 mL of tap water at 37 °C. The basal gastric secretion was collected for 60 min and GSE was administered i.g. in gradual doses ranging from 8 to 64mg/kg, each dose being administered on a separate test day. In control tests, vehicle (1 mL of saline, i g) was given in the same volume as in tests with GSE and the collection of gastric juice was continued for the final 120 min. The volume and acid concentration of each collected sample of gastric juice were measured and acid outputs was calculated and expressed in terms of micromoles of acid per 30 min[12].
Acute gastric lesions were induced by an intragastric (ig) application of 100% ethanol similarly to the method described previously[11,12]. Briefly, 100% ethanol in a volume of 1.5 mL was administered ig to rats by means of a metal orogastric tube. After 60 min, the animals were lightly anesthetized with ether, their abdomen was opened by the midline incision and stomach exposed for the measurement of GBF by means of using the H2-gas clearance technique as described previously[13]. For this purpose double electrodes of electrolytic regional blood flowmeter (Biotechnical Science, Model RBF-2, Osaka, Japan) were inserted into the gastric mucosa. One of these electrodes was used for the local generation of gaseous H2 and another for the measurement of tissue H2.
With this method, the H2 generated locally was carried out by flow of blood, while the polarographic current detector reads out decreasing tissue H2. The tissue H2 clearance curve was used to calculate an absolute flow rate (mL/100 g/min) in the oxyntic area as described previously[13]. The measurements were made in three areas of the mucosa and the mean values of the measurements were calculated and expressed as percent changes of those recorded in the vehicle (saline) treated animals. After the GBF measurement, the stomach was removed, rinsed with water and pinned open for macroscopic examination. The area of necrotic lesions in oxyntic mucosa was determined by computerized planimetry (Morphomat, Carl Zeiss, FRG)[12-14] by the person who did not know to which experimental group the animals belonged.
The WRS was induced in the animals that were placed in restraint cages and immersed vertically to the level of the xiphoid process in a water bath of 23 °C for 3.5 h[13,14]. After 3.5 h of WRS, the rats were lightly anesthetized with ether, the abdomen was opened and the stomach was exposed. The GBF was measured in the oxyntic gland area of the stomach by means of local H2-gas clearance method using an electrolytic regional blood flow meter (Biomedical Science, Model RBF-2, Osaka, Japan) as described above. The stomach was removed, opened along the greater curvature and placed flat to count the number of gastric lesions by two investigators, unaware of the treatment given as described in our previous studies[13,14]. The stress lesions were defined as round or linear mucosal defects of at least 0.1 mm in diameter.
The role of sensory afferent nerves in mechanism of WRS-induced gastric lesions with or without pretreatment with GSE was determined in rats with or without capsaicin induced deactivation of these nerves. For this purpose the animals were pretreated with capsaicin (Sigma Co., St. Louis, MO, USA) injected s.c. for 3 consecutive days at a dose of 25, 50 and 50 mg/kg for about 2 wk, before the experiment[15,16]. All injections of capsaicin were performed under ether anesthesia to counteract the pain reactions and respiratory impairment associated with injection of this agent. To check the effectiveness of the capsaicin denervation, a drop of 0.1 mg/mL solution of capsaicin was instilled into the eye of each rat and the protective counting movements were counted as described previously[16]. Rats with or without capsaicin-denervation received vehicle or GSE (5-80 mg/kg ig) 30 min prior to exposure to 3.5 h of WRS. At the end of WRS, animals were anesthetized and then the GBF and the number of gastric lesions were measured in a similar manner as mentioned above.
In groups of rats exposed to standard 3.5 h of WRS without or with pretreatment with GSE, the mucosal samples from the gastric mucosa were taken by biopsy (about 200 mg) from oxyntic gland area without mucosal lesions immediately after the animals were killed to determine the mucosal generation of PGE2 by radioimmunoassay (RIA) as described previously[17]. The capability of the mucosa to generate PGE2 was expressed in nanograms of wet tissue weight.
Since lipid peroxidation is a well-established mechanism of cellular injury induced by reactive oxygen metabolites, we measured the changes in the MDA as an indicator of this lipid peroxidation in gastric mucosa[18,19] exposed to WRS without and with the treatment with GSE. For the measurement of lipid peroxidation, the tissue was weighed, transferred to the ice-cooled test tube and homogenized in 400 μL of 20 m mol/L Tris buffer pH 7.4 containing 5 mM butylated hydroxytoluene to prevent new lipid peroxidation that can occur during the homogenization. The homogenate was then centrifuged at 4 oC for 10 min and resulted supernatant (200 μL) was stored in -80 oC until an assay of lipid peroxidation. The content of lipid peroxidation was measured at 37 oC by spectrophotometer at a wave length of 586 nm and compared with the absorbance of purified MDA as the standard.
The activity of SOD was measured in the gastric mucosa of rats exposed to WRS with or without pretreatment with GSE using SOD 525 assay kit (OXIS International, Inc., Portland, USA)[19,20]. The gastric tissue was first washed with 0.9% NaCl containing 0.16 mg/mL heparin to remove red blood cells which may interfere with the SOD activity in the gastric specimens. Then the biopsy sample was blotted on the filter paper, weighed and finally homogenized in 400 μL of PBS buffer pH 7.4 using a tissuemizer Ultra Turax (Janke and Kunkel GmbH and Co., Staufen, Germany). The principle of the SOD measurement is based on the SOD-mediated increase in the rate of auto-oxidation of 5, 6, 6a, 11β-tetrahydro-3,9,10-trihydrobenzoflurene in aqueous solution at 37 oC to yield a chromophore with maximum absorbance at 525 nm. Ethanol-chloroform extraction was employed to inactivate Mn-SOD and Fe-SOD and to ensure that the assay is specific for Cu/Zn-SOD.
The expression of SOD was determined by RT-PCR in the gastric mucosa of intact rats or following exposure to WRS with or without GSE. Samples of the gastric oxyntic mucosa (about 500 mg) were scraped off on ice using glass slide and then immediately snap frozen in liquid nitrogen, and stored at -80 oC. Total RNA was isolated from the gastric oxyntic mucosa using a rapid guanidinum isothiocyanate/phenol chloroform single step extraction kit from Stratagene (Stratagene GmbH, Heidelberg, Germany)[21,22]. Following precipitation, the RNA was resuspended in RNase-free TE buffer and the concentration was estimated by absorbance at 260 nm wave length. Samples were frozen at -80 oC until analysis.
First strand cDNA was synthesized from total cellular RNA (5 µg) using 200 U Strata Script TM reverse transcriptase (Stratagene GmbH, Heidelberg, Germany) and oligo (dT) primers (Stratagene GmbH, Heidelberg, Germany). After the reverse transcription, the transcriptase activity was destroyed by heating, and the cDNA was stored at -20 oC until PCR.
A 496-bp fragment of SOD was amplified from single-stranded DNA by PCR using two oligonucleotide primers to SOD. The SOD sense primer was 5’CGAGTTATGGCGACGAAG3’ and antisense primer was 5’GTCAGCAGTCACATTGCC3’. The primers for SOD and β-actin were synthesized by Biometra (Gottingen, Germany). Concomitantly, amplification of control rat β-actin (Clon Tech, Palo Alto, CA, USA) (764 bp) was performed on the same samples to verify RNA integrity.
DNA amplification was carried out under the following conditions; denaturation at 94 oC for 1 min, annealing at 60 oC for 45 s, and extension at 72 oC for 45 s. The number of amplification cycles was 29 for SOD. Each PCR-product (8 µL) was electrophoresed on 1.5% agarose gel stained with ethidium bromide, and then visualized under UV light. Location of predicted PCR product was confirmed by using a 100-bp ladder (Gibco BRL/Life Technologies, Eggenstein, Germany) as standard marker.
The intensity of bands was quantified in a semi-quantitative manner using densitometry (LKB Ultrascan, Pharmacia, Sweden) as described in detail in our previous studies[22]. Briefly the gel was photographed under UV transillumination. The intensity of PCR products was measured using video image analysis system (Kodak Digital Science). The SOD mRNA signals were standardized against the β-actin mRNA signal for each sample and results were expressed as SOD mRNA/β-actin mRNA ratio.
Results are expressed as means±SE. The significance of the difference between means was evaluated using analysis of variance followed by Duncan’s test with a level of significance at P < 0.05.
As shown in Table 1, gastric acid output reached the value of 134±11 μmol/30 min, while fasting plasma gastrin concentration averaged of 53 ± 4 μmol/L in control rats treated with vehicle (saline). GSE applied ig in a dose of 8 mg/kg ig failed to affect significantly the gastric acid output and plasma gastrin levels as compared to those in control animals. GSE applied ig in a dose of 16 mg/kg and higher, produced a significant decrease in the gastric acid output and significantly raised the plasma gastrin levels (Table 1).
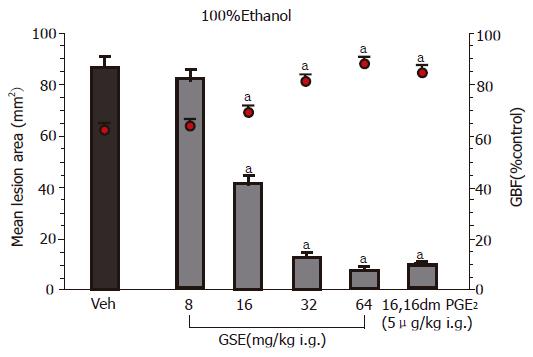
Figure 1 shows the effect of topical pretreatment with GSE applied ig in graded concentrations ranging from 8 to 64 mg/kg on the mean area of gastric lesions induced by 100% ethanol and the accompanying changes in the GBF. Ethanol caused typical widespread gastric lesions with an area of 86±5 mm2 and reduced the GBF by about 38% as compared to that in intact gastric mucosa. Pretreatment with GSE applied in the lowest dose of 8 mg/kg, failed to influence the ethanol lesions but starting from the dose 16 mg/kg up to dose of 64 mg/kg of GSE, a dose-dependent reduction in gastric lesions was observed; the dose that inhibits ethanol damage by 50% (ID50) being 28 mg/kg. This protective effect of GSE applied in graded doses against ethanol damage was accompanied by the significant rise in the GBF. The gastroprotective effect of GSE and accompanying increase in the GBF were similar to those achieved by the pretreatment with methyl analog of PGE2 applied 30 min before the application of ethanol.
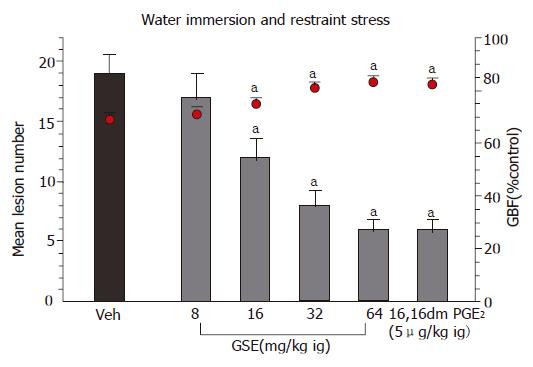
As shown in Figure 2, the pretreatment with GSE applied topically in a doses ranging from 8 to 64 mg/kg caused a significant attenuation of WRS-induced gastric damage; the ID50 being 36 mg/kg. The GBF was significantly decreased in rats exposed to 3.5 h of WRS but GSE given 30 min before the exposure of animals to 3.5 h of WRS resulted in a dose-dependent rise in the GBF as compared to that obtained in vehicle-treated animals (Figure 2).
Table 2 shows the results of determination of MDA+4 HNE as an index of lipid peroxidation in the gastric mucosa or rats exposed to ethanol- or WRS without or with GSE pretreatment. The MDA concentration was significantly increased in vehicle-control gastric mucosa exposed to ethanol or WRS as compared to that in the intact gastric mucosa. Pretreatment with GSE applied ig in a dose 16 mg/kg or higher, significantly attenuated the MDA concentration in animals exposed to ethanol or WRS (Table 2). The mucosal SOD activity was inhibited in the gastric mucosa of animals exposed to ethanol or WRS as compared to the respective value in intact gastric mucosa (Table 2). In contrast, the pretreatment with GSE applied in i.g. in graded doses ranging from 8 up to 64 mg/kg, which caused a significant attenuation of the damage induced by ethanol or WRS, dose-dependently reversed the deleterious effect of ethanol or WRS on the SOD activity and significantly counteracted the ethanol and WRS-induced rise in the MDA content (Table 2).
| Type of test | MDA concentration | SOD activity |
| (nmol/g) | (unit/g) | |
| Intact | 0.5 ± 0.02 | 733 ± 43 |
| Vehicle+ethanol | 14.8 ± 2.3a | 425 ± 32a |
| Vehicle+WRS | 16.8 ± 2.3a | 425 ± 32a |
| GSE (mg/kg i.g.)+ethanol | ||
| 8 | 13.7 ± 2.3 | 438 ± 37 |
| 16 | 10.4 ± 2.8c | 549 ± 38c |
| 32 | 8.3 ± 0.9c | 675 ± 40c |
| 64 | 6.6 ± 0.7c | 695 ± 43c |
| GSE (mg/kg i.g.)+WRS | ||
| 8 | 15.7 ± 2.6c | 438 ± 37c |
| 16 | 13.4 ± 2.3c | 549 ± 38c |
| 32 | 10.3 ± 1.9c | 675 ± 40c |
| 64 | 8.6 ± 0.8c | 695 ± 43c |
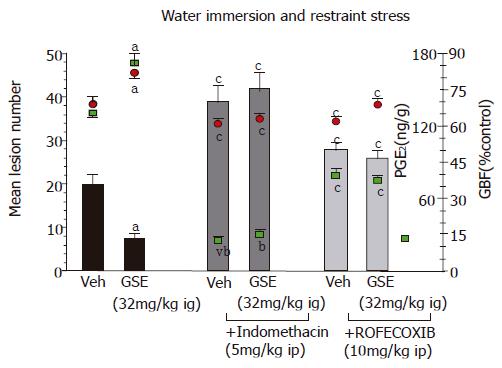
Pretreatment with GSE (32 mg/kg ig) significantly reduced gastric lesions induced by WRS in a manner similar to that presented in Figure 2. The ip administration of indomethacin at a dose of 5 mg/kg which inhibited generation of endogenous PGE2 by about 85%, aggravated the WRS-induced gastric lesions and significantly decreased GBF as compared to the respective values in animals treated with vehicle. Such pretreatment with indomethacin reversed almost completely the protective effect of GSE against WRS-induced lesions and the accompanying increase in the GBF (Figure 3).
The L-NNA, an inhibitor of NO-synthase by itself failed to affect the area of gastric lesions and the fall in the GBF induced by ethanol or WRS (Table 3, Figure 4). Pretreatment with GSE (32 mg/ kg ig) resulted in a significant reduction of ethanol- or WRS-induced gastric damage and significantly decreased the GBF as compared with the respective values attained in rats without GSE administration. Co-treatment with L-arginine (200 mg/kg ig) but not with D-arginine, added to GSE restored the gastroprotection and the accompanying increase in the GBF induced by this extract in L-NNA treated animals.
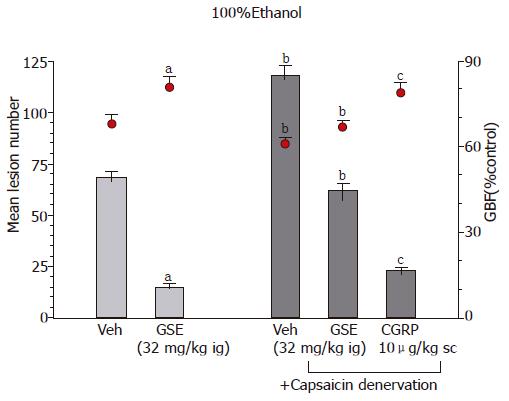
Capsaicin-denervation which by themselves aggravated ethanol- and WRS-induced gastric damage significantly attenuated the GSE-induced protection and hyperemia against lesions induced by ethanol or WRS (Table 4, Figure 5). Co-administration of calcitonine gene-related peptide (CGRP) with GSE in rats with capsaicin-denervation restored the protective and hyperemic effects of this extract against ethanol- and WRS-induced gastric lesions.
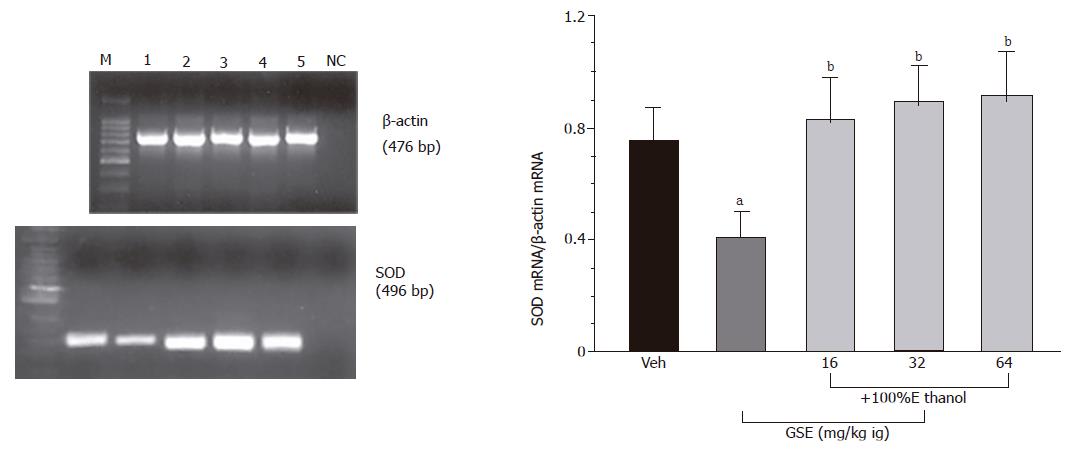
As shown in Figure 6, mRNA for SOD was detected by RT-PCR as a strong signal in the gastric mucosa of vehicle-control animal not treated with ethanol. In contrast, the signal for the SOD mRNA in the gastric mucosa of ethanol-treated rats was faint. The ratio of SOD mRNA over β-actin mRNA was significantly decreased in ethanol-treated animals as compared to those treated with vehicle only. SOD mRNA was strongly expressed in animals pretreated with various doses of GSE (16-64 mg/kg ig) before the ethanol application. The ratio of mRNA for SOD over the β-actin mRNA was significantly increased in gastric mucosa of rats pretreated with graded doses of GSE as compared to that attained in vehicle-pretreated rats that were given ethanol.
This study shows that GSE attenuates the lesions in the rat stomach by the intragastric application of noxious agent such as ethanol or those caused by stress and that this protective effect of GSE is accompanied by the increase in the GBF and SOD expression and its activity and reduction of MDA concentration, that is widely considered as an index of lipid peroxidation. The inhibition of acid-dependent lesions caused by WRS can be attributed to the inhibition of acid secretion by this compound as observed in this study using well-conditioned and fully adapted GF rats. This protective and hyperemic activity of GSE against WRS ulcerogenesis was abolished by COX inhibitors such as indomethacin and rofecoxib and significantly reduced by L-NNA. Thus, we conclude that GSE containing citrous flavonoids exerts a potent gastroprotective activity against ethanol- and WRS-induced gastric lesions and this protective effect in the stomach may involve endogenous PG derived from COX-1 and COX-2 activity, suppression of lipid peroxidation and gastric hyperemia mediated by NO and neuropeptides released from afferent sensory nerves.
Previous studies documented that grapefruit seeds are the major depository for limonoids (triterpenoid dilactones chemically related to limonin) of these 77% are neutral while 2% are acidic limonoids[1-3]. Grapefruit contains also many flavonoids that includes glycosides, naringenin, quercetin, kaempferol, hesperetin and apigenin being the most abundant among their aglycones[4]. GSE, containing flavonoids, has been shown to possess antibacterial, antiviral and antifungal properties[2,3]. This beneficial action of GSE was attributed to the antioxidative activity of grapefruit containing citrus flavonoids[4,23], since, for instance, these flavonoids were found to exhibit the potent anti-H pylori activity in vitro[5,24]. Furthermore, grapefruit containing flavonoids were also recently implicated in cytoprotection against injury induced by algal toxins in isolated hepatocytes[6,25]. Interestingly, GSE in a formulation of Citricidal, was demonstrated to be effective against more than 800 bacterial and vital strains, 100 strains of fungus and a large number of single or multicelled parasites[1]. These antimicrobiological properties against a wide range of Gram-negative and Gram-positive organisms were attributed to the disruption by GSE of the bacterial membrane and the subsequent liberation by this extract of the bacterial cytoplasmic contents within a relatively short time (e.g. 15-20 min). In a similar report, it was reported that GSE-induced antimicrobial action is comparable to that of proven antibacterials[2]. Moreover, naringenin, the bioactive component of GSE, showed anti-cancer activity against human breast cancers[7,26]. The underlying mechanism of the therapeutic efficacy of citrus fruits such as grapefruits and red grapes seems to depend upon the presence of different classes of polyphenolic flavonoids that were shown to inhibit platelet aggregation, thus decreasing the risk of coronary thrombosis and myocardial infarction[27-30].
The involvement of GSE in the mechanism of gastric mucosal defense against the formation of gastric lesions caused by obnoxious substances has been little studied. Herein, we provide evidence that GSE applied topically caused dose-dependent diminution of acute gastric lesions induced by both, 100% ethanol and WRS. Previous studies demonstrated that the damaging action of ethanol and WRS could be attributed to the enhancement in the reactive oxygen substances (ROS) and the ROS-dependent increase in the lipid peroxidation and inhibition of antioxidizing enzyme activity[18,19,21]. We found that GSE dose-dependently attenuated the rise in MDA content in the gastric mucosa injured by ethanol or WRS indicating that this extract can attenuate the process of lipid peroxidation implicated in the pathogenesis of ethanol and WRS-induced gastric damage[18,19]. Moreover, we have demonstrated that ethanol decreased the gene expression and activity of SOD in the gastric mucosa suggesting that the suppression of key mucosal antioxidizing enzyme along with the elevation of lipid peroxidation, play an important role in the pathogenesis of these lesions. This increase in the mucosal lipid peroxidation as well as the fall in SOD expression and its activities were attenuated by GSE, suggesting that the reduction in lipid peroxidation by this seed extract may contribute to the attenuation of the deleterious effect of noxious agents on the gastric mucosa. This is supported by the fact that the GBF was elevated and gastric PGE2 production were enhanced in animals treated with GSE as compared to those treated with vehicle. Our finding is in accordance with observations that some flavonoids stimulated PGE2 production by isolated gastric mucosal cells while suppressing gastric acid secretion via direct inhibitory effect on H+/K+-ATPase activity[24]. It is not concluded that NO/NOS system is also involved in gastroprotection against ethanol and WRS caused by GSE because both, GSE-induced protection and hyperemia were counteracted by L-NNA, a non-specific inhibitor of NO-synthase and this effect was restored in these animals by the combined treatment with L-arginine and GSE. Thus, our study implies that some natural products of the citrous fruits such as GSE, afford protection against ethanol and stress-induced gastric damage due to endogenous PG and the preservation of expression and activity of a major antioxidizing enzyme such as SOD.
The mechanism of gastroprotective activity of GSE appears to be dependent on endogenous PG and the functional activity of sensory nerves releasing CGRP. This notion is supported by our findings that indomethacin, a non-selective inhibitor of COX-1 and COX-2 activity or functional ablation of sensory afferent nerves by capsaicin reversed GSE-induced protection and accompanying hyperemia. In addition, co-treatment of exogenous CGRP with GSE, administered to replace the deficit of this peptide in capsaicin-treated animals, restored the protective efficacy of GSE. Interestingly, rofecoxib which is a highly selective COX-2 inhibitor[17], also attenuated the gastroprotective and hyperemic activities of GSE suggesting the involvement of COX-2 derived products in the gastroprotection and increase in the GBF induced by this extract. Further studies assessing the mRNA expression of COX-1 and COX-2 in GSE-treated mucosa should reveal which enzymatic pathway is involved in aforementioned effects of this extract.
This gastroprotective activity of the GSE could be attributed to naringenin, a major GSE flavonoid, because this flavonoid was reported to exhibit gastroprotection against the gastric injury induced by absolute ethanol, predominantly due to the increase in the mucus secretion[10]. It is of interest that this gastroprotective effect of naringenin and accompanying increase in the mucus secretion, were, in part, attenuated by indomethacin, supporting the contribution of endogenous PG to the mechanism of gastroprotection by grapefruit products[10]. As shown in our present study, GSE by itself enhanced the gastric mucosa generation of PGE2 but our unpublished evidence indicates that this extract can be also effective against aspirin-induced gastric lesions under the conditions, where PG generation is completely suppressed. Therefore, it seems likely, that endogenous PG might not be primary mediators of this protection and other gastroprotective factors such as NO and/or neuropeptides released from sensory afferent nerves could be involved. This was the reason for carrying out the study with L-NNA, a potent NO-synthase inhibitor and with capsaicin, applied in a dose that causes functional ablation of sensory nerves releasing vasoactive neuropeptides such as CGRP. We found that L-NNA and capsaicin denervation inhibited the GSE-induced protection against ethanol- and WRS-induced gastric lesions and accompanying gastric mucosal hyperemia. Our study militates against PG as the primary mediator in the mechanism of GSE-induced gastroprotection and this remains in accordance with our previous report that meciadanol, the synthetic flavonoid inhibiting the activity of HDC, prevented the ethanol- and aspirin-induced injury in rat stomach without altering the mucosal generation of prostacyclin (PGI2)[9].
Another candidate involved in the GSE-induced protection could be gastrin, which is known to exhibit both, gastroprotective and hyperemic activities[11]. Indeed, we addressed this issue by direct determination of the plasma gastrin levels by specific RIA and we found that gastrin is elevated in GSE-treated animals. This effect could be secondary to the inhibition of gastric acid secretion caused by this compound as demonstrated in our study by the administration of GSE to the chronic, well-adapted rats with chronic GF. Suppression of gastric secretion by GSE might contribute to the protective activity of this extract against WRS, because WRS damage depends upon gastric acidity and becomes exaggerated by acidic conditions in the stomach.
In summary, we have demonstrated in this report that the pretreatment with GSE reduces the ethanol and WRS-induced gastric damage through the preservation of the antioxidizing enzyme (SOD) activity, reduction of free radical-dependent lipid peroxidation, enhancement in the GBF and plasma gastrin levels. Endogenous PG appears to be important mediator of this protection, but other mediators such as NO and neuropeptides released from sensory nerves such as CGRP could be also involved in the gastroprotective and hyperemic activities of this extract. No study, so far has been undertaken to examine the ulcer healing efficacy of GSE, but the fact that this grapefruit seed extract exerts a potent anti-H pylori in vitro[30] and profound gastroprotective effects in laboratory animals warrants a further approach for its potential application in healing of chronic ulcerations in human beings.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Heggers JP, Cottingham J, Gusman J, Reagor L, McCoy L, Carino E, Cox R, Zhao JG. The effectiveness of processed grapefruit-seed extract as an antibacterial agent: II. Mechanism of action and in vitro toxicity. J Altern Complement Med. 2002;8:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Reagor L, Gusman J, McCoy L, Carino E, Heggers JP. The effectiveness of processed grapefruit-seed extract as an antibacterial agent: I. An in vitro agar assay. J Altern Complement Med. 2002;8:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Proteggente AR, Pannala AS, Paganga G, Van Buren L, Wagner E, Wiseman S, Van De Put F, Dacombe C, Rice-Evans CA. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic Res. 2002;36:217-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 402] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Tirillini B. Grapefruit: the last decade acquisitions. Fitoterapia. 2000;71 Suppl 1:S29-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Bae EA, Han MJ, Kim DH. In vitro anti-Helicobacter pylori activity of some flavonoids and their metabolites. Planta Med. 1999;65:442-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Blankson H, Grotterød EM, Seglen PO. Prevention of toxin-induced cytoskeletal disruption and apoptotic liver cell death by the grapefruit flavonoid, naringin. Cell Death Differ. 2000;7:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr Cancer. 1996;26:167-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 314] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Keevil JG, Osman HE, Reed JD, Folts JD. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J Nutr. 2000;130:53-56. [PubMed] |
| 9. | Konturek SJ, Kitler ME, Brzozowski T, Radecki T. Gastric protection by meciadanol. A new synthetic flavonoid inhibiting histidine decarboxylase. Dig Dis Sci. 1986;31:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Motilva V, Alarcón de la Lastra C, Martín MJ. Ulcer-protecting effects of naringenin on gastric lesions induced by ethanol in rat: role of endogenous prostaglandins. J Pharm Pharmacol. 1994;46:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Konturek SJ, Brzozowski T, Bielanski W, Schally AV. Role of endogenous gastrin in gastroprotection. Eur J Pharmacol. 1995;278:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Brzozowski T, Konturek SJ, Kwiecien S, Pajdo R, Drozdowicz D, Sliwowski Z, Muramatsu M. SU-840, a novel synthetic flavonoid derivative of sophoradin, with potent gastroprotective and ulcer healing activity. J Physiol Pharmacol. 1998;49:83-98. [PubMed] |
| 13. | Brzozowski T, Konturek PC, Sliwowski Z, Drozdowicz D, Hahn EG, Konturek SJ. Importance of nitric oxide and capsaicin-sensitive afferent nerves in healing of stress lesions induced by epidermal growth factor. J Clin Gastroenterol. 1997;25 Suppl 1:S28-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Konturek SJ, Brzozowski T, Konturek PK, Majka J, Dembiński A. Role of salivary glands and epidermal growth factor (EGF) in gastric secretion and mucosal integrity in rats exposed to stress. Regul Pept. 1991;32:203-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Holzer P, Livingston EH, Saria A, Guth PH. Sensory neurons mediate protective vasodilatation in rat gastric mucosa. Am J Physiol. 1991;260:G363-G370. [PubMed] |
| 16. | Brzozowski T, Konturek SJ, Sliwowski Z, Pytko-Polończyk J, Szlachcic A, Drozdowicz D. Role of capsaicin-sensitive sensory nerves in gastroprotection against acid-independent and acid-dependent ulcerogens. Digestion. 1996;57:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Drozdowicz D, Stachura J, Pajdo R, Hahn EG. Role of prostaglandins generated by cyclooxygenase-1 and cyclooxygenase-2 in healing of ischemia-reperfusion-induced gastric lesions. Eur J Pharmacol. 1999;385:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53:39-50. [PubMed] |
| 19. | Kwiecień S, Brzozowski T, Konturek PC, Pawlik MW, Pawlik WW, Kwiecień N, Konturek SJ. The role of reactive oxygen species and capsaicin-sensitive sensory nerves in the pathomechanisms of gastric ulcers induced by stress. J Physiol Pharmacol. 2003;54:423-437. [PubMed] |
| 20. | Kwiecień S, Brzozowski T, Konturek PC, Pawlik MW, Pawlik WW, Kwiecień N, Konturek SJ. Gastroprotection by pentoxyfilline against stress-induced gastric damage. Role of lipid peroxidation, antioxidizing enzymes and proinflammatory cytokines. J Physiol Pharmacol. 2004;55:337-355. [PubMed] |
| 21. | Brzozowski T, Konturek P, Konturek SJ, Kwiecień S, Sliwowski Z, Pajdo R, Duda A, Ptak A, Hahn EG. Implications of reactive oxygen species and cytokines in gastroprotection against stress-induced gastric damage by nitric oxide releasing aspirin. Int J Colorectal Dis. 2003;18:320-329. [PubMed] |
| 22. | Konturek PC, Duda A, Brzozowski T, Konturek SJ, Kwiecien S, Drozdowicz D, Pajdo R, Meixner H, Hahn EG. Activation of genes for superoxide dismutase, interleukin-1beta, tumor necrosis factor-alpha, and intercellular adhesion molecule-1 during healing of ischemia-reperfusion-induced gastric injury. Scand J Gastroenterol. 2000;35:452-463. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Lee YS, Reidenberg MM. A method for measuring naringenin in biological fluids and its disposition from grapefruit juice by man. Pharmacology. 1998;56:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Beil W, Birkholz C, Sewing KF. Effects of flavonoids on parietal cell acid secretion, gastric mucosal prostaglandin production and Helicobacter pylori growth. Arzneimittelforschung. 1995;45:697-700. [PubMed] |
| 25. | Kanno S, Shouji A, Asou K, Ishikawa M. Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. J Pharmacol Sci. 2003;92:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Guthrie N, Carroll KK. Inhibition of mammary cancer by citrus flavonoids. Adv Exp Med Biol. 1998;439:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418-425. [PubMed] |
| 28. | Folts JD. Potential health benefits from the flavonoids in grape products on vascular disease. Adv Exp Med Biol. 2002;505:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Osman HE, Maalej N, Shanmuganayagam D, Folts JD. Grape juice but not orange or grapefruit juice inhibits platelet activity in dogs and monkeys. J Nutr. 1998;128:2307-2312. [PubMed] |
| 30. | Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |