Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.551
Revised: January 4, 2004
Accepted: February 24, 2004
Published online: January 28, 2005
AIM: To observe the effect of cholecystokinin (CCK) on lipofusin value, neuronal dendrite and spine ultrastructure, and total cellular protein during the process of experimental neuronal aging.
METHODS: Experimental neuronal aging study model was established by NBA2 cellular serum-free culture method. By using single intracellular lipofusin value from microspectrophotometry, morphology of neuronal dendrites and spines from the scanner electron microscopy, and total cellular protein as the indexes of experimental neuronal aging, we observed the effect of CCK8 on the process of experimental neuronal aging.
RESULTS: Under the condition of serum-free culture, intracellular fluorescence value (%) increased with the extension of culture time (1 d 8.51±3.43; 5 d 10.12±3.03; 10 d 20.54±10.3; 15 d 36.88±10.49; bP<0.01). When CCK was added to serum-free culture medium, intracellular lipofusin value (%) decreased remarkably after consecutive CCK reaction for 10 and 15 d (control 36.88±10.49; 5 d 32.03±10.01; 10 d 14.37±5.55; 15 d 17.31±4.80; bP<0.01). As the time of serum-free culturing was prolonged, the number of neuronal dendrite and spine cells decreased. The later increased in number when CCK8 was added. CCK8 could improve the total cellular protein in the process of experimental neuronal aging.
CONCLUSION: CCK8 may prolong the process of experimental neuronal aging by maintaining the structure and the number of neuronal dendrite and spine cells and changing the total cellular protein.
- Citation: Sun XJ, Lu QC, Cai Y. Effect of cholecystokinin on experimental neuronal aging. World J Gastroenterol 2005; 11(4): 551-556
- URL: https://www.wjgnet.com/1007-9327/full/v11/i4/551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.551
Aging is a universal phenomenon, and the common feature of all cellular organisms. Finding the reasons for aging and the ways to postpone its process has theoretical implications and extensive application value.
Neuronal aging is a core subject in geriatrics. As the neuronal aging is relatively long, it is difficult to conduct in vitro experiments. On the contrary, an in vivo experiment, which is relatively easy to control its experimental condition and its short cycle of cell culture, is considered a realistic method of neuronal aging study.
Mice neuroblastoma cell culture is widely recognized by scholars as an experimental neuronal model in organic chemistry, immunochemistry, pharmacology, and neuropeptide researches. Cai et al[1] devised an experimental neuronal aging model by culturing mice neuroblastoma cells under the condition of serum-free culture. This model is easy to use due to avoidance of the influence of serum.
Neuropeptide is a kind of internal active substances inside the brain. It is speculated that there are probably 200 kinds of neuropeptides existing in the mammal brain and 60% synaptic connections are related to neuropeptides. Some of the neuropeptides can function both as transmitters and modulators. CCK includes CCK8, CCK39 and CCK33. Pro-CCK gives rise to CCK8 within the brain.CCK, which is one kind of neurotransmitters[2], is commonly found in the central nervous system[3,4]. It not only regulates food absorption and body weight[5], but also plays an important role in sensitivity[6], reflection[7], growth, learning[8], emotion, memory[9], adaptive development and brain plasticity[10].
Some research findings have revealed that neuropeptide is remarkably low in brain and cerebrospinal fluid (CSF) of the aged people, especially those who suffer from diseases in relation to aging, such as Alzheimer’s disease (AD), Parkinson’s disease (PD)[11], etc. The cholinergic neurons in cerebral cortex degenerate severely in AD, while CCK could protect and slow the degenerative process of cholinergic neurons[12,13]. There are degeneration and loss of substantia nigra dopamine(DA), decreased DA neurotransmitters in PD patients while CCK could regulate and increase DA transmitter[14] and its activity[15]. These results suggest that CCK may regulate and strengthen neuronal aging process. This study was to observe CCK influence on some biological indexes in neuronal aging process by a neuronal aging model established using serum-free culture of mice neuroblastoma cell line.
Neuroblastoma A2 (NBA2) from American Type Culture Collection was a gift from Professor Larry Davis of University of New Mexico School of Medicine.
Beef serum (Shanghai Biological Experimental Cell Biological Technology Company), Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies USA), insulin (Shanghai Biochemical Drug Factory), trypsin, beef serum albumin and [Tyr-So3H27]- cholecystokinin fragment 26-33 amide (CCK8 No.C-9271, Sigma) were used in our study.
Culture medium with serum (BD) contained 10% beef serum DMEM, NaHCO3 3.7 g/L, gentamicin 50000 u/L. Serum-free culture medium (AID): beef serum albumin 500 mg, insulin 5 mg, gentamicin 50000 u were added into 1 liter DMEM. Culture medium with peptides (AID -CCK): CCK8 was added into AID culture medium, CCK8 concentration was 10-7 mol/L.
Equipments used included Nikon phase reversal contrast microscope, LNA-III type CO2 culture box, Hitachi low temperature freezer and JSM-840 scan electron microscope (These equipments are all made in Japan). UMSP-30 microspectrometry and its MOP-Videoplan image analysis system were products of OPTON in Germany. FACStar Plus type fluency cytometer was produced by Becton Dickinson in USA.
This model was established by Yan Cai and described as follows. Preparation of cell samples: NBA2 cells were inoculated into a culture bottle containing a 10 mm×20 mm slide inside, cultured for 24 h in 50 mL/L CO2 at 37 °C with BSD culture medium. The original culture medium was discarded and cells were rinsed 3 times with Hanks solution, then AID culture medium was added, replaced with fresh AID culture medium every other day, cells were inoculated every several d, and the first set of cultured cells was collected on d 1, 5, 10, and 15, and tested on the same day.
Based on the method in our laboratory[1], inoculated cells were assigned to AID group and CCK group randomly. The CCK experimental group was subdivided into 5-d CCK, 10-d CCK, and 15-d CCK groups according to the different reaction periods of CCK. The incubation time for both control and experimental groups was 15 d. BSD solution in experimental group was replaced and the cells were washed 3 times with Hanks solution, then AID-CCK medium was added for incubation. AID-CCK was changed every 2 d. The old solution was discarded when CCK action reached the pre-determined date, serum-free solution was added and stayed for 15 d for test. One hundred percent reference standard was set by 1% fluorescence intensity on green plastic slides. Lipofusin value was randomly tested in 30 cells from each group. By using a computer, data on these cells were collected for statistical analysis.
The cells after 3 d of secondary culture were digested by 0.125% trypsin and collected and calculated through BD culture solution. Then, 106 cells were inoculated into medium containing a 10 mm×10 mm cover slide, incubated for 24 h under the condition of 50 mL/L CO2 at 37 °C, washed 3 times with Hanks solution. The cover slide was taken out randomly from BD culture solution and fixed by 2% glutaraldehyde. The rest of the cells were divided randomly into serum-free and CCK groups, AID or AID- CCK medium was added respectively. The cover slide with attached cells was taken out and fixed by 2% glutaraldehyde. The cells were washed 3 times with phosphate buffer solution and fixed for 1 h by 1% osmic acid, dehydrated by achohol, stained by uranium isoamyl acetate, dried by marginal place and coated by gold using ion sputter-coater and then observed by scanning electron microscope manufactured by JEOL of Japan.
FD, AID and CCK8-AID solutions were added to culture NBA2 cells. After 1, 5, 10 d, single cell suspension was made by the cells grown into layers at the concentration of 6×106/mL. The cells were fixed by a final concentration of 75% alchohol for 24 h. Protein was stained by 1ug/mL FITC, analysed by flow cytometer. The equipment was the FACS tar plus produced by Becton Dickinson Co. Ltd. USA, argon (Ar) laser activator was used as laser light, at output power 200 mW, activator wave 488 nm. The fluorescence was tested by 585 filter. The total protein content in 3000-5000 cells was measured for each group. Data were input into MP310 computer and the mean, median, mode and the peak of cellular total proteins were obtained by CELL Fit software of Becton Dickinson Co. Ltd.
Statistic analysis of lipofusin fluorescence value was based on POMS analysis of variance software.
The accumulated intracellular fluorescence lipofusin value increased as the time of serum-free culture increased, reaching the highest value on d 15 (P<0.01) (Table 1).
CCK8 action for 10 and 15 d could decrease intracellular lipofusin value significantly (Table 2).
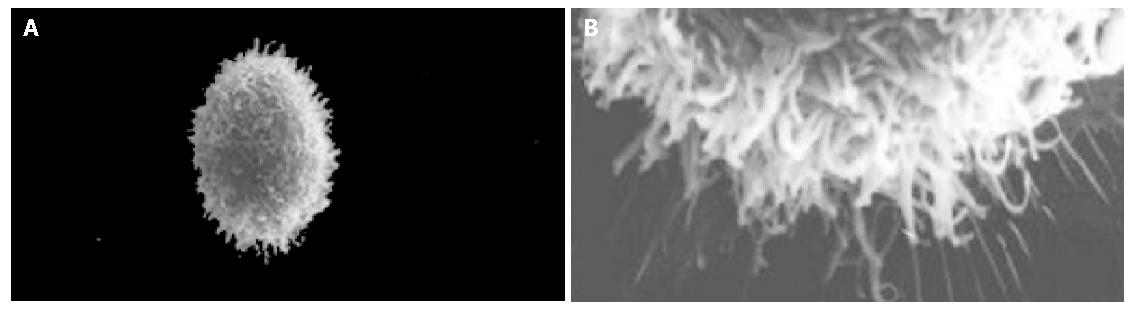
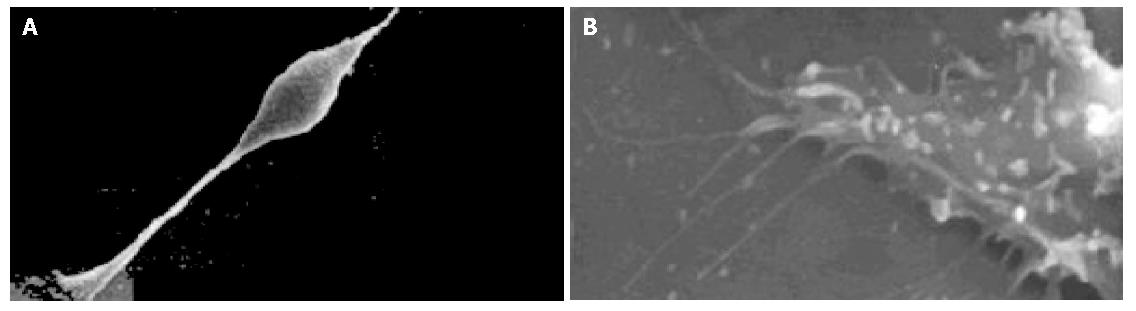
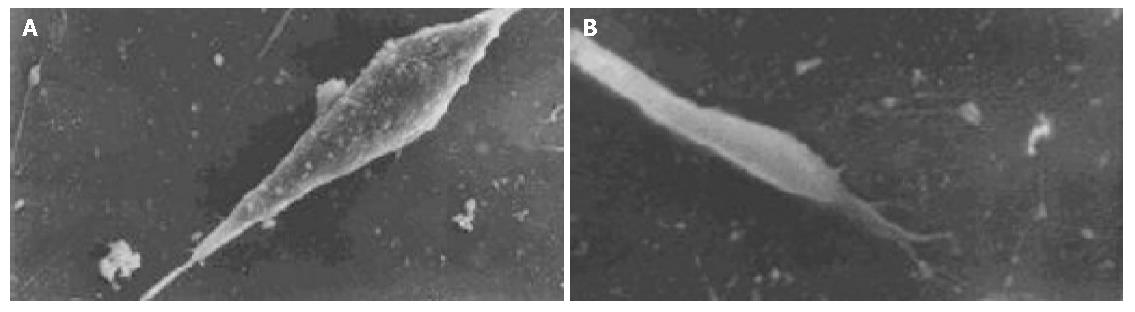
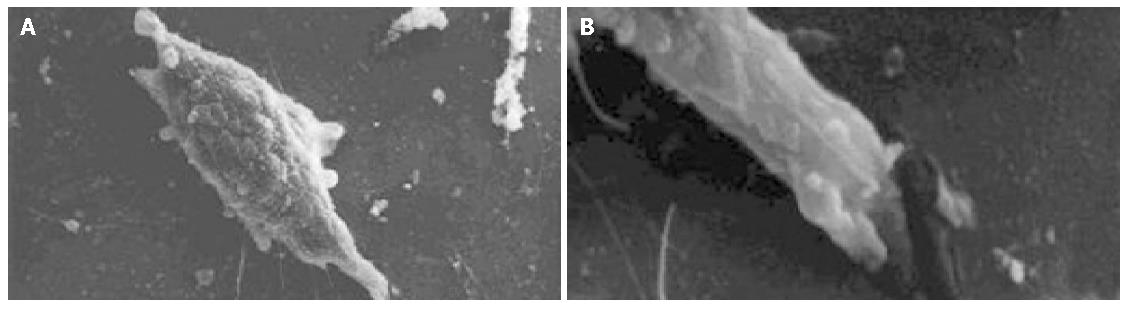
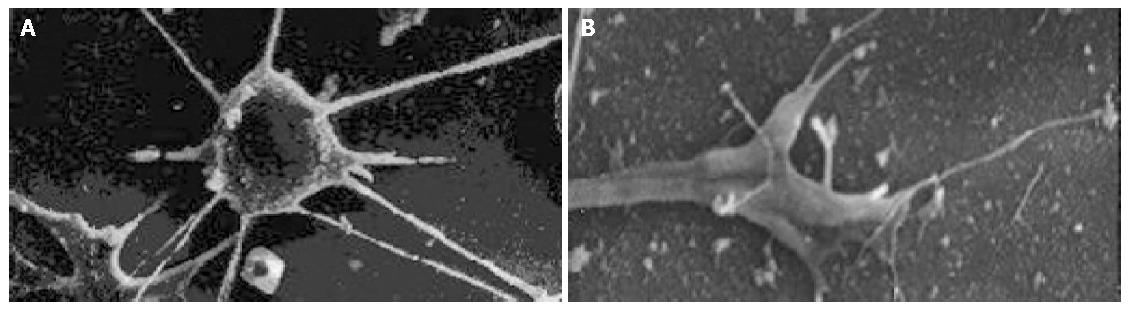
The cell body of the cells cultured with serum solution turned round and oval, and dense microvilli were observed (Figure 1A). Microvilli shaped like hassock were evenly distributed, 4-6 μm in length and projected to all directions and some of them were curl-like (Figure 1B). On d 5 of serum-free incubation, cells turned spindle or triangle. Dendrites were grown with a lot of spines at its terminal (Figure 2A). The spines projected peripherally with ends split, at 3-5 μm in length (Figure 2B). On d 10 of serum-free culture, the nodi from the surface of the cells were getting bigger (Figure 3A), the dendrites of the local cell were bigger and the number of the spines was fewer at 1-2 μm in length (Figure 3B). On d 15 of serum-free culture, the nodi on the surface of cells were maximal and the dendrites were getting smaller (Figure 4A). The ends of the dendrites were deadwood like and spines disappeared (Figure 4B).
On d 5 of CCK8 action, cells looked star-like with long and more dendrites, and more spines appeared at both ends of each dendrite. On d 15, nodal granules (particles) on cell surface were smaller than those in serum-free culture group (Figure 5A). A number of spines could be found at the end of each dendrite(Figure 5B).
On d 1 of non-serum incubation, the mean, median, mode and peak of total cellular protein all decreased remarkably. On d 5 of serum-free incubation, the mean, median, mode of total cellular protein all increased, while the peak significantly decreased. On d 10 of serum-free incubation, the mean, median, mode and peak of total cellular protein increased (Table 3).
| Group | Days of culture | Cell number | Total cellular protein (channel) | |||
| Mean | Median | Mode | Peak | |||
| BD | 1 | 5000 | 487 | 469 | 358 | 13 |
| AID | 2837 | 355 | 349 | 345 | 7 | |
| BD | 5 | 5000 | 575 | 562 | 561 | 44 |
| AID | 5000 | 676 | 651 | 631 | 31 | |
| BD | 10 | 5000 | 501 | 497 | 513 | 23 |
| AID | 5000 | 626 | 620 | 629 | 26 | |
On d 1 of CCK8 action, the mean, median, mode and peak of total cellular protein increased. On d 5 of CCK8 action, there was an increase in the mode but a decrease in the peak. On d 10 of CCK8 action, the mean, median, mode and peak dropped remarkably (Table 4).
| Group | Day of culture | Cell number | Total cellular protein (channel) | |||
| Mean | Median | Mode | Peak | |||
| AID | 1 | 2837 | 355 | 349 | 345 | 7 |
| CCK8+AID | 5000 | 582 | 605 | 665 | 7 | |
| AID | 5 | 5000 | 676 | 651 | 631 | 31 |
| CCK8+AID | 5000 | 543 | 452 | 553 | 28 | |
| AID | 10 | 5000 | 626 | 620 | 629 | 26 |
| CCK8+AID | 5000 | 502 | 471 | 508 | 17 | |
Cai et al[1] designed 5 different kinds of culture medium for establishing an experimental neuronal aging model, and they found that serum-free culture medium consisting of insulin, albumin, DMEM could maintain an equilibrium and even standstill status after a period of growing time of NBA2 cells. As the culture time extended, differentiation appeared, which is helpful for the observation of cellular aging. Our experiment adopted AID as serum-free culture medium.
Generally speaking, lipofusin is a residual substance produced by autophagocytosis of the improper structure and function of subcellular components by lysosomes. This element is accumulated as age increases[16]. There is a great deal of lipofusin in aged human beings, animals and AD animal model[17,18]. The yellow pigment ultrastructure is covered by heterogeneous membrane and contains high-density molecules and vesicles of lipid. It is a biological marker indicating the decline of the whole cell function, and that may be the etiology of the neuronal loss. Its deposit may cause changes of cellular components, such as the amount of cellular liquid and the number of mitochondria, decrease of roughly surfaced endoplasmic reticulum (RER), simplification of the Golgi complex and empty vesicle formation. Lipofusin is the result of aging, and may generate auto-fluorescence and can be measured by spectrometry.
The experimental results suggested that intracellular liopfusin fluorescence value dropped to the lowest on d 1, and the intracellular lipofusin fluorescence value increased considerably after every 5 d as the culture time extended, and reached the highest on d 15 (P<0.01). The tendency towards increase in accumulation of the intracellular fluorescence value in NBA2 is parallel to the increase of neuronal intracellular fluorescence value along with increase of age in mammals and humans. Therefore, the intracellular fluorescence value of lipofusin can be used as a good index for experimental neuronal aging model.
Lipofusin fluorescence value by continuous action of CCK8 of 10 and 15 d groups was much lower than that in control group (P<0.01), but still higher than serum-free culture 1 d group, indicating that CCK8 could lower the speed of accumulation of lipofusin but could not prevent the generation of lipofusin. This suggests that CCK8 could postpone the process of experimental neuronal aging but could not prevent the general tendency of experimental neuronal aging.
Every point of the whole dendrite could connect with the terminal of axon from other neurons and generate synapse. A variety of the dendrites of neurons have microvilli known as dendritic spines, which especially connect with synapse and conduct neuronal action. The morphological changes related to aging include the decrease of the brain volume and weight and enlargement of ventricles, the etiology of which was attributed partly to the loss of neurons. The loss of process and synapse of the aging brain is in selected region rather than in the whole brain[19]. The pathological changes in aging brain are the generation of neuronal tangle[20] and senile plague[21,22] and degeneration and loss of neurons[23], dendrites[24], spines[25] and synapses[26]. Experimental results suggest dendrites were generated on d 5 of serum-free incubation and different kinds of dendrites and spines were grown. As the incubation time extended, the number of dendrites and spines decreased and later they even disappeared. The shape of dendrites was damaged. These changes are similar to the morphology of the aging and AD brain and indicate NBA2 cells grow and become mature through division and multiplication and differentiation. Maturity and aging reflect the process of neuronal aging. Under the action of CCK8, cells generate more dendrites as well as spines. Morphologically dendrites and spines remained intact on d 15, demonstrating that CCK8 promotes the growth of dendrites and spines of experimental neurons, by maintaining the structure integrity of dendrites and spines. In addition, CCK8 could delay degeneration and disappearance of dendrites and spines.
Protein is the material foundation of life. Protein is not only the major component of cell tissue but also participates in most physical activities. Most of the physiological functions in the body are fulfilled through protein, and protein always plays a key role in it. Therefore, life is a special motion of the protein. In elderly people, aged animals and aging related disorders such as AD and PD, the quantity of proteins could change remarkably in brain tissue and CSF. Some researchers have found that the quantity of amyloid precursor protein (APP)[27], abeta 40 and abeta 42[28], Tau proteins[29,30] is increased, while cytochrome C oxidase[31], growth-associated protein-43[32], neurosin[33] and calcium binding protein calbindin-D (28K)[34] are decreased in brain tissue and CSF of the elder people, aged animals and AD patients. These increased proteins related to aging and aging correlated diseases are neurotoxic proteins. The increase of these proteins could lead to neurological aging and characteristic changes of AD disease such as senile plague and neuritic tangles. The decreased proteins in aging and AD patients are enzyme or neuron growth factors essential to metabolism in the body. The decrease of these proteins affects the normal metabolism of neurons and finally leads to death of the neurons, indicating that total cellular protein could change remarkably, and is accompanied with aging process. It needs further investigation to identify the specific kind of protein changes.
Our results suggest that CCK8 changes the total cellular protein of the experimental neuronal aging and decreases the generation and accumulation of intracellular lipofusin, promotes the growth of dendrites and spines and delays their degeneration. Hence, CCK8 protects the aging process of experimental neuronal aging.
Edited by Wang XL and Zhu LH
| 1. | Cai Y, Shen JK, Lu RH. An experimental model for the study of the aging of neurons: serum-free culture of mouse neuroblastoma cells. Shiyan Shengwu Xuebao. 1985;18:453-461. |
| 2. | Rehfeld JF, Lindberg I, Friis-Hansen L. Increased synthesis but decreased processing of neuronal proCCK in prohormone convertase 2 and 7B2 knockout animals. J Neurochem. 2002;83:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Sohal VS, Cox CL, Huguenard JR. Localization of CCK receptors in thalamic reticular neurons: a modeling study. J Neurophysiol. 1998;79:2820-2824. [PubMed] |
| 4. | Fratucci De Gobbi JI, De Luca LA, Johnson AK, Menani JV. Interaction of serotonin and cholecystokinin in the lateral parabrachial nucleus to control sodium intake. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1301-R1307. [PubMed] |
| 5. | Plagemann A, Rake A, Harder T, Melchior K, Rohde W, Dörner G. Reduction of cholecystokinin-8S-neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Neurosci Lett. 1998;258:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Manni L, Lundeberg T, Tirassa P, Aloe L. Cholecystokinin-8 enhances nerve growth factor synthesis and promotes recovery of capsaicin-induced sensory deficit. Br J Pharmacol. 2000;129:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Funakoshi K, Nakano M, Atobe Y, Kadota T, Goris RC, Kishida R. Selective projections of cholecystokinin-8 immunoreactive fibers to galanin immunoreactive sympathetic preganglionic neurons in a teleost, Stephanolepis cirrhifer. Neurosci Lett. 2001;316:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Löfberg C, Harro J, Gottfries CG, Oreland L. Cholecystokinin peptides and receptor binding in Alzheimer's disease. J Neural Transm. 1996;103:851-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Hansen TV, Nielsen FC. Regulation of neuronal cholecystokinin gene transcription. Scand J Clin Lab Invest Suppl. 2001;234:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Pisu MB, Conforti E, Scherini E, Bernocchi G. Gastrin-cholecystokinin immunoreactivity in the central nervous system of Helix aspersa during rest and activity. J Exp Zool. 2000;287:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Fernandez A, de Ceballos ML, Jenner P, Marsden CD. Striatal neuropeptide levels in Parkinson's disease patients. Neurosci Lett. 1992;145:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Takahashi M, Sugaya K, Kojima K, Katoh T, Ueki M, Kubota K. SUT-8701, a cholecystokinin analog, prevents the cholinergic degeneration in the rat cerebral cortex following basal forebrain lesioning. Jpn J Pharmacol. 1993;61:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Sugaya K, Takahashi M, Kubota K. Cholecystokinin protects cholinergic neurons against basal forebrain lesion. Jpn J Pharmacol. 1992;59:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Xie JX, Tang M, Zhang C. Effect of cholecystokinin-8 microinjection into ventral tegmental area on dopamine release in nucleus accumbens of rats: an in vivo voltammetric study. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Smolnik R, Fischer S, Hagenah J, Kis B, Born J, Vieregge P. Brain potential signs of slowed stimulus processing following cholecystokinin in Parkinson's disease. Psychopharmacology (Berl). 2002;161:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Lasn H, Winblad B, Bogdanovic N. The number of neurons in the inferior olivary nucleus in Alzheimer's disease and normal aging: A stereological study using the optical fractionator. J Alzheimers Dis. 2001;3:159-168. [PubMed] |
| 17. | Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H. Neuropathology of Alzheimer's disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci. 1999;249 Suppl 3:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 244] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Transm Suppl. 1998;53:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 307] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Anderton BH. Ageing of the brain. Mech Ageing Dev. 2002;123:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Hall GF, Lee S, Yao J. Neurofibrillary degeneration can be arrested in an in vivo cellular model of human tauopathy by application of a compound which inhibits tau filament formation in vitro. J Mol Neurosci. 2002;19:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Teter B, Ashford JW. Neuroplasticity in Alzheimer's disease. J Neurosci Res. 2002;70:402-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Marcinkiewicz M. BetaAPP and furin mRNA concentrates in immature senile plaques in the brain of Alzheimer patients. J Neuropathol Exp Neurol. 2002;61:815-829. [PubMed] |
| 23. | van de Nes JA, Sandmann-Keil D, Braak H. Interstitial cells subjacent to the entorhinal region expressing somatostatin-28 immunoreactivity are susceptible to development of Alzheimer's disease-related cytoskeletal changes. Acta Neuropathol. 2002;104:351-356. [PubMed] |
| 24. | Ohm TG, Münch S, Schönheit B, Zarski R, Nitsch R. Transneuronally altered dendritic processing of tangle-free neurons in Alzheimer's disease. Acta Neuropathol. 2002;103:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease. Brain Cogn. 2002;49:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Grace EA, Rabiner CA, Busciglio J. Characterization of neuronal dystrophy induced by fibrillar amyloid beta: implications for Alzheimer's disease. Neuroscience. 2002;114:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. Overexpression of Alzheimer's disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem. 2002;277:44670-44676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Roher AE, Weiss N, Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry. 2002;41:11080-11090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Askanas V, Engel WK. Newest pathogenetic considerations in inclusion-body myositis: possible role of amyloid-beta, cholesterol, relation to aging and to Alzheimer's disease. Curr Rheumatol Rep. 2002;4:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Kapaki E, Paraskevas GP, Zalonis I, Zournas C. CSF tau protein and beta-amyloid (1-42) in Alzheimer's disease diagnosis: discrimination from normal ageing and other dementias in the Greek population. Eur J Neurol. 2003;10:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Cottrell DA, Borthwick GM, Johnson MA, Ince PG, Turnbull DM. The role of cytochrome c oxidase deficient hippocampal neurones in Alzheimer's disease. Neuropathol Appl Neurobiol. 2002;28:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Mori N, Morii H. SCG10-related neuronal growth-associated proteins in neural development, plasticity, degeneration, and aging. J Neurosci Res. 2002;70:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Mitsui S, Okui A, Uemura H, Mizuno T, Yamada T, Yamamura Y, Yamaguchi N. Decreased cerebrospinal fluid levels of neurosin (KLK6), an aging-related protease, as a possible new risk factor for Alzheimer's disease. Ann N Y Acad Sci. 2002;977:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Geula C, Bu J, Nagykery N, Scinto LF, Chan J, Joseph J, Parker R, Wu CK. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 2003;455:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |