Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.6014
Revised: March 1, 2004
Accepted: March 2, 2004
Published online: October 14, 2005
AIM: To investigate the expression of receptor-binding cancer antigen expressed on SiSo cells (RCAS1) in metastatic lymph nodes from gastrointestinal cancer.
METHODS: Metastatic lymph nodes from gastrointestinal cancer were detected for RCAS1 by immunohistochemical staining and mRNA in situ hybridization.
RESULTS: A total of 102 metastatic lymph nodes from bile duct, gastric, colon, and pancreatic cancer were investigated for RCAS1 expression. The immunoreactivity of RCAS1 was identified in 100% of metastatic lymph nodes. Both local and distant metastatic lymph nodes showed RCAS1 expression. On the contrary, specimens of non-cancerous lymph nodes were negative for RCAS1. The result of mRNA in situ hybridization was also confirmed by the finding of immunohistochemical staining. RCAS1 mRNA was detected in all tumor cells that metastasized to lymph nodes.
CONCLUSION: All metastatic lymph nodes express RCAS1 in tumor cells at both protein and mRNA levels, and RCAS1 should be used as a complementary factor for identification of metastatic lymph nodes from gastrointestinal cancers.
- Citation: Leelawat K, Engprasert S, Tujinda S, Suthippintawong C, Enjoji M, Nakashima M, Watanabe T, Leardkamolkarn V. Receptor-binding cancer antigen expressed on SiSo cells can be detected in metastatic lymph nodes from gastrointestinal cancers. World J Gastroenterol 2005; 11(38): 6014-6017
- URL: https://www.wjgnet.com/1007-9327/full/v11/i38/6014.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i38.6014
One of the major factors for the poor prognosis of gastrointestinal cancer patients is lymph node metastasis[1-3]. To indicate the lymph node metastasis, pathologists must carefully identify tumor cells spreading to lymph nodes in each serial section of pathological specimens. The suitable method for the identification of tumor cells in lymph nodes is to stain the specimen with antibody, which highly recognizes the tumor antigen. At this time, the antibody to cytokeratin is widely used for detection of lymph node metastasis from breast cancer[4,5]. However,a current study showed that the sensitivity of cytokeratin to lymph node metastasis from gastrointestinal cancer is low[6].
Recently, receptor-binding cancer antigen expressed on SiSo cells (RCAS1) is recognized as type II membrane protein and secreted as a soluble protein[7,8]. This tumor-associated antigen has been demonstrated in several kinds of gastrointestinal cancer by immunohistochemical study using the specific mouse mAb, 22-1-1. The result of RCAS1 expression was evaluated for the percent incidence in various cancers including gall bladder cancer (70%)[9] cholangiocarcinoma (85.9-96.4%)[10,11] gastric cancer (54.3%)[12] pancreatic cancer (100%)[13] and colorectal cancer (100%)[14]. However, until now, there has been no intensive study about the expression of RCAS1 in lymph node metastasis from gastrointestinal cancer. Therefore, the purpose of the present study was to investigate the expression of RCAS1 at both protein and mRNA levels in lymph node metastasis from gastrointestinal cancer.
Fifteen specimens of non-cancerous lymph nodes and 102 specimens of metastatic lymph nodes from different gastrointestinal cancers (surgically resected in Rajavithi Hospital, Bangkok, Thailand) were collected for RNA investigation and immunohistochemical staining. The study was carried out with the approval of the Ethical Committee of Rajavithi Hospital. Pathological diagnosis and evaluation were routinely performed.
The sections (4 μm) were deparaffinized and immunostained for RCAS1 as described previously[14]. Briefly, the sections were incubated overnight at 4°C with 1:1 000 dilution of 22-1-1 mouse mAb (Medical & Biological Laboratories Co., Ltd.). Biotinylated rabbit anti-mouse IgM (DAKO) was applied to the sections followed by avidin-biotin-peroxidase conjugate (ABC Elite, Vector Labs). The immunohistochemical reactions were developed with freshly prepared 3,3-diaminobenzidine tetrahydrochloride solution (Histofine SAB-PO kit) and counterstained with hematoxylin. As a negative control, primary antibody was replaced with mouse IgM. The positive cell staining in each slide was identified under a high power field (400) of Olympus BH2 microscope (field width = 0.5 mm).
Fluorescent probe preparation RCAS1 cDNA fragments from metastatic lymph nodes were synthesized by RT-PCR as described previously[14] using forward primer 5-ACCTTACTGCCCTCCGTCTA-3 and reverse primer
5-CTTCTTCATTAGCCGTTGTG-3. The nucleotide sequence was confirmed with a Model 310 genetic analyzer (PE Biosystems) using a BigDye terminator cycle sequencing kit and used for generating fluorescent probes using Gene ImagesTM random prime labeling kit (Amersham, Biosciences). Twenty nanograms per microliter of denatured RCAS1 cDNA was mixed with 10 mL of nucleotide mix, 5 mL of primer, and 1 mL of 5 U/mL Klenow enzyme solution. The reaction was incubated at 37 ? for 1 h and stored at -20 °Cuntil used.
In situ hybridization The specimens were deparaffinized in xylene. The sections were treated with proteinase K for 20 min at 37 °C then diluted fluorescent-RNA probe (1 ng/mL with hybridization buffer) was applied onto each specimen. The slides were incubated at 55 °C for 2 h. The sections were rinsed with TBS and incubated for 15 min with blocking mixture (10% fetal calf serum). For detection of mRNA, the sections were incubated at room temperature for 30 min with anti-FITC/alkaline phosphatase. The immunoreactions were visualized by incubating the sections at room temperature with substrates and immersed in tap water for 5 min. The slides were counterstained with hematoxylin before observation under a light microscope.
Statistical analysis for comparing the expression of RCAS1 in the specimens was performed using SPSS 10.0 for Windows.P<0.05 was considered statistically significant.
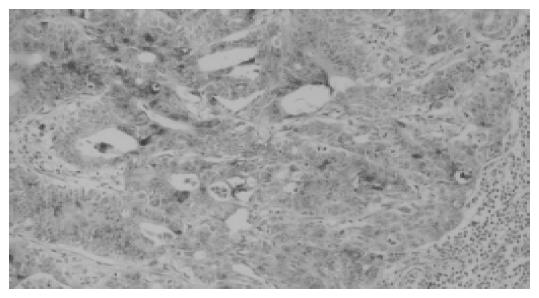
A total of 102 metastatic lymph nodes from various kinds of cancer were investigated for RCAS1 expression as shown in Table 1. The metastatic lymph nodes were divided into two groups (local and distant metastatic nodes) according to the TNM staging systems. The immunostaining pattern for RCAS1 was observed in the cytoplasm of tumor cells but not in lymphocytes (Figure 1). Most metastatic tumor cells showed a high intensity of RCAS1 immunostaining. The staining level was not related with tumor stage or differentiation status. All sections in negative control (non-cancerous lymph nodes) showed no immunoreactivity. RCAS1 was identified in 100% of metastatic lymph nodes. Interestingly, several primary cancers, which identified RCAS1 expression in some parts of tumor tissues, were found to express RCAS1 in most of the metastatic tumor cells in lymph nodes.
| Type of cancer | Total No. of lymph nodes | No. of lymph nodes express RCASI(%) | |
| Local metastasis | Distance metastasis | ||
| Bile duct cancer | 10 | 4 | 14(100) |
| Colon cancer | 56 | 6 | 62(100) |
| Pancreatic cancer | 12 | 2 | 14(100) |
| Stomach cancer | 10 | 2 | 12(100) |
The RCAS1 cDNA of metastatic lymph nodes from various gastrointestinal cancers was determined by reverse-transcription-PCR. The 802 bp product was sequenced and its homology was compared with the RCAS1 cDNA sequence databases present at NCBI GenBank (gi: 13528905). This cDNA fragment started from nucleotide 41 to 842 of RCAS1 cDNA. As demonstrated in Figure 2, the cDNA fragment from metastatic lymph nodes revealed a high similarity throughout the 802 bp in the coding region.
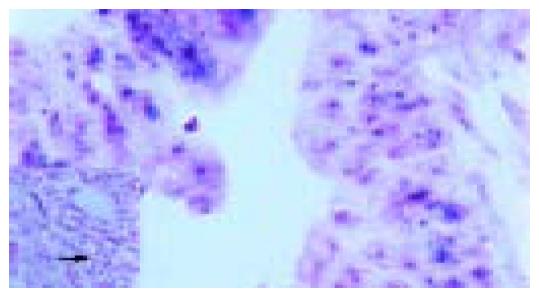
To confirm further the expression of RCAS1 in metastatic lymph nodes, RCAS1 mRNA in situ hybridization was performed using the cDNA probes showing a high similarity with RCAS1 cDNA. The expression of RCAS1 mRNA was clearly identified in cytoplasm of tumor cells but not in lymphocytes. Figure 3 represents the typical RCAS1 mRNA expression, which could be detected in cytoplasm of tumor cells and represented the negative signal for RCAS1 mRNA in lymphocytes.
RCAS1 expression in lymph node metastasis from various gastrointestinal cancers was first explored at protein level by immunohistochemical staining. The RCAS1 cDNA obtained from metastatic lymph nodes from various gastrointestinal tumors showed high homology with the RCAS1 cDNA sequence databases present at NCBI GenBank (gi: 13528905). This finding increases the reliability of in situ hybridization for identification RCAS1 of mRNA expression in metastatic tumor cells.
As suspected, it was expressed in all cases of metastatic lymph nodes. RCAS1 was shown to be a ligand for a putative receptor on immune cells such as T cells and natural killer cells[7-14]. Scientists suggest that RCAS1 may play a protective role in tumor cells against immune surveillance by inhibition of clonal expression and inducing receptor-expressing cell death. It has been previously reported that activated lymphocytes process receptors for RCAS1[7]. Thus, binding interaction between them induces apoptosis of lymphocytes. The mechanism by which tumor cell expressed RCAS1 mediates lymphocyte apoptosis is a main biologic pathway to escape the immune surveillance in both local and distant metastatic lymph nodes.
Although previous results[13,14] have demonstrated the correlation between the staining level and tumor stage or differentiation status, this study did not find any significant correlation between them. Most metastatic tumor cells showed a high intensity of RCAS1 immunostaining. This result may explain why tumor cells expressing RCAS1 metastasize the lymph nodes. The role of RCAS1 in lymph node metastasis needs further study.
Since the expression of RCAS1 is found in gastrointestinal cancers and their metastatic lymph nodes, it can be used as an additional step for identification of the status of lymph nodes.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L, Liu YX. Correlation of tumor-positive ratio and number of perigastric lymph nodes with prognosis of patients with surgically-removed gastric carcinoma. World J Gastroenterol. 2004;10:182-185. [PubMed] |
| 2. | Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 996] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 3. | Ichikura T, Ogawa T, Chochi K, Kawabata T, Sugasawa H, Mochizuki H. Minimum number of lymph nodes that should be examined for the International Union Against Cancer/American Joint Committee on Cancer TNM classification of gastric carcinoma. World J Surg. 2003;27:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Lara JF, Young SM, Velilla RE, Santoro EJ, Templeton SF. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long-term follow-up. Cancer. 2003;98:2105-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Euhus DM. Cytokeratin staining and other sentinel node controversies. Clin Breast Cancer. 2003;4 Suppl 1:S49-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Mukai M, Sato S, Nakasaki H, Tajima T, Saito Y, Nishiumi N, Iwasaki M, Tokuda Y, Ogoshi K, Inoue H. Occult neoplastic cells in the lymph node sinuses and recurrence of primary breast, lung, esophageal, and gastric cancer. Oncol Rep. 2004;11:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Nakashima M, Sonoda K, Watanabe T. Inhibition of cell growth and induction of apoptotic cell death by the human tumor-associated antigen RCAS1. Nat Med. 1999;5:938-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Sonoda K, Nakashima M, Kaku T, Kamura T, Nakano H, Watanabe T. A novel tumor-associated antigen expressed in human uterine and ovarian carcinomas. Cancer. 1996;77:1501-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Oshikiri T, Hida Y, Miyamoto M, Hashida H, Katoh K, Suzuoki M, Nakakubo Y, Hiraoka K, Shinohara T, Itoh T. RCAS1 as a tumour progression marker: an independent negative prognostic factor in gallbladder cancer. Br J Cancer. 2001;85:1922-1927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Enjoji M, Nakashima M, Nishi H, Choi I, Oimomi H, Sugimoto R, Kotoh K, Taguchi Ki, Nakamuta M, Nawata H. The tumor-associated antigen, RCAS1, can be expressed in immune-mediated diseases as well as in carcinomas of biliary tract. J Hepatol. 2002;36:786-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Suzuoki M, Hida Y, Miyamoto M, Oshikiri T, Hiraoka K, Nakakubo Y, Shinohara T, Itoh T, Okushiba S, Kondo S. RCAS1 expression as a prognostic factor after curative surgery for extrahepatic bile duct carcinoma. Ann Surg Oncol. 2002;9:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Fukuda K, Tsujitani S, Maeta Y, Yamaguchi K, Ikeguchi M, Kaibara N. The expression of RCAS1 and tumor infiltrating lymphocytes in patients with T3 gastric carcinoma. Gastric Cancer. 2002;5:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Akashi T, Oimomi H, Nishiyama K, Nakashima M, Arita Y, Sumii T, Kimura T, Ito T, Nawata H, Watanabe T. Expression and diagnostic evaluation of the human tumor-associated antigen RCAS1 in pancreatic cancer. Pancreas. 2003;26:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Leelawat K, Watanabe T, Nakajima M, Tujinda S, Suthipintawong C, Leardkamolkarn V. Upregulation of tumour associated antigen RCAS1 is implicated in high stages of colorectal cancer. J Clin Pathol. 2003;56:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |