Published online Sep 14, 2005. doi: 10.3748/wjg.v11.i34.5377
Revised: December 14, 2004
Accepted: December 19, 2004
Published online: September 14, 2005
AIM: To investigate the effects of entero-hepatic bile acid circulation on the inter-digestive migrating myoelectrical complex (MMC) in rats.
METHODS: Thirty-two rats were divided into four groups. Three pairs of bipolar silver electrodes were chronically implanted in the antrum, duodenum and jejunum. Three groups of them were ligated around the upper part of common bile duct (CBD). The experiments were performed in conscious and fasting state. The gastrointestinal myoelectrical activity was recorded. Ursodeoxycholic acid (UDCA) and saline were then perfused into stomachs of two groups with CBD obstruction and the effects of them on the MMC were observed.
RESULTS: A typical pattern of MMC was observed in normal fasting rats. MMC of antral and duodenal origin disappeared temporarily in earlier stage of CBD obstruction. While MMC of jejunum origin appeared. increased MMC cycle duration was seen after 4 d in rats with CBD obstruction. The MMC after CBD obstruction was characterized by an increased duration of phase II-like activity and decreased duration of phase I & III activity. Perfusion into stomachs with UDCA resulted in a shorter MMC cycle duration and a longer duration of phase III of duodenal origin compared to the normal group.
CONCLUSION: Entero-hepatic bile acid circulation initiates inter-digestive MMC of duodenal origin.
- Citation: Fang P, Dong L, Zhang WJ, Luo JY. Relationship between entero-hepatic bile acid circulation and interdigestive migrating myoelectrical activity in rats. World J Gastroenterol 2005; 11(34): 5377-5380
- URL: https://www.wjgnet.com/1007-9327/full/v11/i34/5377.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i34.5377
Inter-digestive motility has been physically studied intensively[1-3], but mechanism is still unclear. Inter-digestive motility of gastrointestinal tract is characterized by a typical cycling complex of myoelectric activity. The migrating myoelectrical complex (MMC) is a distinct pattern of electromechanical activity observed in gastrointestinal tract during fasting. It serves as a housekeeping role and sweeps residual undigested materials through the digestive tract. Phase III MMC originates at variable sites in the gut from the esophagus to the proximal ileum, especially from antrum and duodenum[2,3].
Previous studies showed that entero-hepatic bile acid circulation and inter-digestive motility should be associated with each other[4]. Bile flow increases during phase II activity and reaches peak just before start of the MMC in the duodenum. It has been thought to exert an important regulatory function on the MMC[5]. Furthermore, phase III MMC plays an important role in the transport of bile acids from the proximal duodenum to the distal small intestine, where bile acids are absorbed for transport back to the liver. But use of different animal species and different methods has led to conflicting results[4-6].
In this article, we describes a new model which was used to further elucidate the role of bile acids in regulating MMC in unrestrained rats without the bias of anesthesia and post-operative influence on gastrointestinal motility. Therefore, the present study aimed to investigate the effects of common bile duct obstruction and UDCA perfusion into stomachs on the inter-digestive myoelectrical activity.
Thirty-two healthy Sprague-Dawley rats (20 males and 12 females) weighing 200-250 g were fed with a dry food, with free access to tap water. After fasting for one night, the rats were intraperitoneally anesthetized with sodium pentobarbital (30 mg/kg). The hair on the skull and abdomen was shaved. The surgery was done under strict aseptic conditions. The abdominal musculature and peritoneum were opened through the linea alba. Three bipolar insulated silver electrodes made of Teflon-coated wire (outer diameter 0.5 mm, length 20 cm) were implanted into the muscular layer of the bowel with a needle as a trocar. One milliliter of the wire was exposed near the implanted end, and the distance between pairs of electrodes should be 2.0-3.0 mm. The electrodes were placed on the gastric antrum 5 mm proximal to the pylorus, the duodenum and jejunum respectively 1 cm and 15 cm distal to the pylorus. The bundled electrode wires were grasped by the clamp through a silastic tube (diameter 2.4 mm), which was then passed through the subcutaneous tunnel from the abdominal incision to the back of the shoulder exit. Furthermore, 24 of them were ligated around the upper part of common bile duct (CBD). During operation, the intestine was kept moist with 9 g/L sterile NaCl solution, and 4 mL of this solution was used intraperitoneally to compensate for intraoperative fluid loss before closure of the abdomen. The abdominal wall was closed in three layers with running Vicryl 4-0 sutures. The rats were kept in a humidified room at 37°C for 2 to 4 h after operation. Then, they were individually housed with free access to water and food. Housing conditions were at 22°C, 60% humidity and a 12 h light/dark cycle for 1 wk before experiment.
The rats were fasted for 12 h with free access to water. The gastrointestinal myoelectrical activity in conscious rats was recorded by a computerized, multichannel recorder (RM-6280C, Chengdu, China). Myoelectrical activity was sampled at a frequency of 1 kHz. The signals were amplified and bandpass was filtered (above 0.3 Hz and below 100 Hz were cut off). The amplitudes of contractions were recorded as μV.
The rats were coded and randomly divided into four groups (groups A, B, C and D), 8 in each. Group A had implanted electrode; group B had implanted electrodes +CBD obstruction; group C had implanted electrodes +CBD obstruction+UDCA perfusion; group D had implanted electrodes +CBD obstruction+saline perfusion. Three days after operation, we started to measure the gastrointestinal motility every day for 3 d. During experiment, the gastrointestinal myoelectrical activity in each rat was recorded for 2 h at least. On the 4th d after operation, before the MMC of duodenal origin recovered, UDCA (Pharmaceutical Factory of Changzhou City) was dissolved immediately before use in 3 mL normal saline at a dose of 20 mg/kg and then perfused into stomachs of one group with implanted electrodes and CBD obstruction. After UDCA was used, the gastrointestinal myoelectrical activity in the rats was continuously recorded for more than 2 h. As a control, normal saline was given in another group with implanted electrodes and CBD obstruction. After the conversion from analog to digital, the signals were stored on optical disk for later analysis.
Data are shown for duodenum. All parameters such as MMC cycle duration, duration of phase III, mean amplitude and frequency of phase III were expressed as mean±SD unless otherwise stated. P < 0.05 was considered statistically significant. One way analysis of variance was performed to compare the means of all variables among groups using software package SPSS 10.0. A comparison was made between two groups using LSD method. Variables could be transformed when they were not coincident with each other.
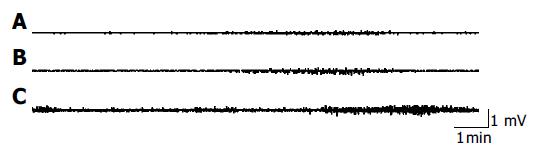
The gastrointestinal motility pattern in rats could mainly be divided into inter-digestive and digestive stage. The inter-digestive stage was characterized by cyclical phase III contractions of the migrating motor complex which occurred in the stomach and duodenum and migrated to the small intestine. The digestive state was characterized by sustained contractions in the gastric antrum and small intestine. In our experiments, a typical pattern of myoelectrical activity in the fasting state appeared gradually in all rats with implanted electrodes about 3 d after operation. The phases of the MMC were as follows: phase I, motor quiescence; phase II, a period of irregular contractile activity; and phase III, a period of rhythmic contractions. The migrating motor complex pattern was disrupted by feeding, and irregular contractions were sustained in the antrum, duodenum and jejunum for 30 min after feeding (Figures 1 and 2).
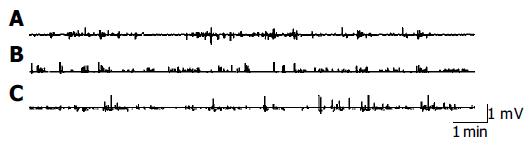
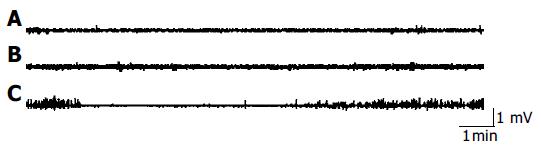
Early after CBD obstruction regular MMC of duodenal origin disappeared, myoelectric activity was characterized by a continuous, irregular, low amplitude phase II-like activity without migrating activity fronts (Figure 3), while MMC of jejunum origin appeared. On the fourth day after CBD obstruction, MMC of duodenal origin started to recover gradually with a lower frequency. Five days after CBD obstruction, 74% of the MMC originated in the jejunum and 26% of the MMC originated in the duodenum. The MMC cycle duration of duodenal origin in CBD obstruction group was obviously longer than that in the group with implanted electrodes. The increased MMC duration was mainly caused by increase of phase II-like activity. But the duration of phase III in CBD obstruction group was not significantly different from that of the group with implanted electrodes. There was no statistical difference between the MMC characteristics of jejunal origin in CBD obstruction group and the group with implanted electrodes.
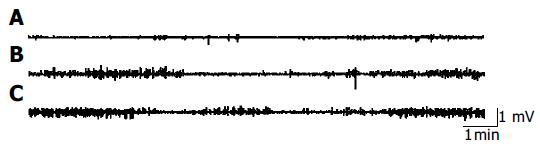
Four days after control recordings were obtained in rats with CBD obstruction and no MMC of duodenal origin was observed. UDCA (20 mg/kg) was dissolved in 3 mL saline and perfused into stomachs of rats. The recording was continued for additional 2 h. The effects of UDCA on the inter-digestive gastrointestinal myoelectrical activity were established after the stomach was perfused with UDCA. Administration of UDCA led to a restart of duodenal MMC. The shorter MMC cycle duration and the longer duration of phase III of duodenal origin were observed. There was no significant difference in the other parameters of MMC of duodenum between the two groups (Figure 4, Table 1). Furthermore, there was no change in the interdigestive gastrointestinal myoelectrical activity of rats with CBD obstruction after normal saline treatment.
| Group | MMCcycleduration/s | Durationof phaseIII/s | Amplitudeof phaseIII/μV | Frequency of phase III/(bursts/min) |
| A | 730.5 ± 44.71 | 163.5 ± 19.1 | 288.4 ± 21.9 | 11.2 ± 1.6 |
| B | 1 680.9 ± 89.5a | 175.5 ± 23.3 | 270.5 ± 31.2 | 10.8 ± 1.5 |
| C | 658.5 ± 42.7ac | 228.8 ± 36.5ac | 279.6 ± 19.8 | 11.6 ± 1.4 |
| D | 1 529.5 ± 75.8ae | 172.5 ± 20.5e | 272.6 ± 28.9 | 10.9 ± 1.8 |
The gastrointestinal motility pattern in rats can be divided into the inter-digestive and digestive states. The inter-digestive state is characterized by cyclical phase III contractions which occurs in antrum and duodenum and migrates to the small intestine. The digestive stage is characterized by sustained, irregular contractions in antrum and small intestine. These two phenomena were observed in our experiments. The migrating motor complex pattern could be disrupted by feeding.
The regulating mechanism of MMC is not completely understood[7-9]. Previous study showed that entero-hepatic bile acid circulation influences MMC and exerts an important regulatory function on MMC[10-12]. Our experiment showed that when entero-hepatic bile acid circulation was interrupted, spontaneous MMC in duodenum disappeared in early CBD obstruction. MMC originated mostly in the jejunum and migrated to the terminal ileum as a compensating mechanism. The duodenum showed continuous phase II-like activity. During the next 4 d after CBD obstruction, MMC of duodenal origin recovered gradually with a longer MMC cycle duration. The increased duration of MMC was mainly the result of an increase of phase II-like activity. UDCA perfusion into stomach resulted in a shorter MMC cycle duration and a longer duration of phase III of duodenal origin. But normal saline did not influence the MMC in rats with CBD obstruction. The underlying mechanisms for the absence of duodenal MMC during the interruption of entero-hepatic circulation are unknown. The possible explanation is that the development of MMC of duodenal origin is not autonomous but dependent on the stimulation of bile acids to the duodenum or the increased pressure in biliary tract[13]. Nieuwenhuijs et al[14] thought that increased pressure in the biliary tract disrupts MMC early after CBD obstruction and the absence of bile in the duodenum and small intestine lengthens the MMC cycle. Ozeki et al[15] thought that duodenum is not a spontaneous MMC oscillator which keeps resting below its oscillation threshold till a stimulus causes oscillation. However, the resting level of the duodenal MMC oscillator is close enough to its exciting threshold and sometimes may exhibit spontaneous oscillation. Artificial drainage of duodenal bile acids could also lead to increase of MMC cycle and phase II-like activity in healthy volunteers[16]. Thus, stimulation of higher bile acid concentration to the mucosa of duodenum in a short time is the prerequisite for the initiation of duodenal origin MMC[17].
Whether entero-hepatic bile acid circulation and intragastric bile salt infusion influence the MMC is still controversial. Some researchers supported the idea that there are no special effects of bile acids on inter-digestive MMC[18]. Luiking et al[19] did not find any change of gallbladder emptying and small intestinal MMC when they perfused artificial bile into duodenum of healthy volunteers during inter-digestive stage. Van Felius et al[20] found that prolongation of the MMC causes pancreatitis, and ligation of the proximal CBD near the bifurcation does not prolong the MMC, suggesting that pancreatitis influences the MMC cycle and hepatic biliary secretion alone does not play a primary role in the regulation of MMC. Some studies showed that initiation of MMC is closely related to the increasing speed of bile acids in plasma. This may explain the conflicting results in the above researches[21,22].
Our model is suitable for studying the possible relationship between entero-hepatic bile acid circulation and inter-digestive gastrointestinal motility. At first, we ligated the CBD near the entrance to the duodenum. Almost all the rats died of pancreatitis. Ligation of the proximal CBD did not influence the function of pancreas. Post-operative gastrointestinal myoelectrical motility was measured in conscious state after the rats recovered from operation and could move freely. The advantages of this model are as follows. The surgical procedures are well tolerated by the animals. Experiments can be performed in unrestrained rats without the bias of sedative drugs, anesthesia and stress of operation. Ligation of the proximal CBD does not influence the function of the pancreas.
In conclusion, our model has made it easier and more precise to study the effects of CBD obstruction and UDCA on MMC in unrestrained rats without the bias of anesthetic agents or post-operative effects. Our findings indicate that that the entero-hepatic bile acid circulation initiates the inter-digestive MMC of duodenal origin, which provides a new therapy for patients with gut motility disorders.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Luiking YC, Akkermans LM, van der Reijden AC, Peeters TL, van Berge-Henegouwen GP. Differential effects of motilin on interdigestive motility of the human gastric antrum, pylorus, small intestine and gallbladder. Neurogastroenterol Motil. 2003;15:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Wang L, Zhou L, Tian R. Role of the area postrema of medulla oblongata in the regulation of canine interdigestive migrating motor complex. Chin Med J (Engl). 2002;115:384-388. [PubMed] |
| 3. | Tanaka T, Kendrick ML, Zyromski NJ, Meile T, Sarr MG. Vagal innervation modulates motor pattern but not initiation of canine gastric migrating motor complex. Am J Physiol Gastrointest Liver Physiol. 2001;281:G283-G292. [PubMed] |
| 4. | Hellström PM, Nilsson I, Svenberg T. Role of bile in regulation of gut motility. J Intern Med. 1995;237:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Romański KW, Salomon E, Borodulin-Nadzieja L, Janocha A. Fluctuations of gastrin, motilin and total bile acid levels in porcine blood before and after feeding. Folia Med Cracov. 2002;43:5-12. [PubMed] |
| 6. | Qvist N, Oster-Jørgensen E, Pedersen SA, Rasmussen L, Hovendal C, Holst JJ. Increases in plasma motilin follow each episode of gallbladder emptying during the interdigestive period, and changes in serum bile acid concentration correlate to plasma motilin. Scand J Gastroenterol. 1995;30:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Suzuki H, Mochiki E, Haga N, Shimura T, Itoh Z, Kuwano H. Effect of duodenectomy on gastric motility and gastric hormones in dogs. Ann Surg. 2001;233:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Spencer NJ, Bywater RA. Enteric nerve stimulation evokes a premature colonic migrating motor complex in mouse. Neurogastroenterol Motil. 2002;14:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Plaza MA. 5-hydroxytryptamine and the gastrointestinal migrating motor complex. Curr Opin Investig Drugs. 2001;2:539-544. [PubMed] |
| 10. | Kajiyama Y, Irie M, Enjoji A, Ozeki K, Ura K, Kanematsu T. Role of bile acids in duodenal migrating motor complexes in dogs. Dig Dis Sci. 1998;43:2278-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Van Ooteghem NA, Van Erpecum KJ, Van Berge-Henegouwen GP. Effects of ileal bile salts on fasting small intestinal and gallbladder motility. Neurogastroenterol Motil. 2002;14:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Einarsson C, Ellis E, Abrahamsson A, Ericzon BG, Bjorkhem I, Axelson M. Bile acid formation in primary human hepatocytes. World J Gastroenterol. 2000;6:522-525. [PubMed] |
| 13. | Utsunomiya N, Tanaka M, Ogawa Y, Konomi H, Takahata S, Nabae T, Yokohata K, Chijiiwa K. Pain associated with phase III of the duodenal migrating motor complex in patients with postcholecystectomy biliary dyskinesia. Gastrointest Endosc. 2000;51:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Nieuwenhuijs VB, Luiking YC, Verheem A, vanBerge-Henegouwen GP, Gooszen HG, Akkermans LM. Disrupted bile flow affects interdigestive small bowel motility in rats. Surgery. 1997;122:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Ozeki K, Sarna SK, Condon RE, Chey WY, Koch TR. Enterohepatic circulation is essential for regular cycling of duodenal migrating motor complexes in dogs. Gastroenterology. 1992;103:759-767. [PubMed] |
| 16. | Nilsson I, Svenberg T, Hedenborg G, Lördal M, Hellström PM. Inhibition of the migrating motor complex by duodenal drainage in man. Neurogastroenterol Motil. 1995;7:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Portincasa P, Peeters TL, van Berge-Henegouwen GP, van Solinge WW, Palasciano G, van Erpecum KJ. Acute intraduodenal bile salt depletion leads to strong gallbladder contraction, altered antroduodenal motility and high plasma motilin levels in humans. Neurogastroenterol Motil. 2000;12:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Neubrand MW, Dominguez-Munoz JE, Reichel C, Kampmann S, Eschmann K, von Falkenhausen M, Bregulla M, Malfertheiner P, Sauerbruch T. Effect of intraduodenal administration of ursodeoxycholic acid on interdigestive interaction between gallbladder motility, pancreatic secretion and endocrine activity. Digestion. 2004;69:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Luiking YC, Kloppers NJ, Roelofs JM, Nieuwenhuijs VB, Peeters TL, Akkermans LM, van Berge Henegouwen GP. Effects of intraduodenal bile on interdigestive gastrointestinal and gallbladder motility in healthy subjects. Digestion. 2001;63:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Van Felius ID, Akkermans LM, Bosscha K, Verheem A, Harmsen W, Visser MR, Gooszen HG. Interdigestive small bowel motility and duodenal bacterial overgrowth in experimental acute pancreatitis. Neurogastroenterol Motil. 2003;15:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | van Ooteghem NA, Moschetta A, Rehfeld JF, Samsom M, van Erpecum KJ, van Berge-Henegouwen GP. Intraduodenal conjugated bile salts exert negative feedback control on gall bladder emptying in the fasting state without affecting cholecystokinin release or antroduodenal motility. Gut. 2002;50:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Andersen PV, Mortensen J, Oster-Jørgensen E, Rasmussen L, Pedersen SA, Qvist N. Cholecystectomy in patients with normal gallbladder function did not alter characteristics in duodenal motility which was not correlated to size of bile acid pool. Dig Dis Sci. 1999;44:2443-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |