Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.5025
Revised: December 18, 2004
Accepted: December 21, 2004
Published online: August 28, 2005
AIM: To investigat the relation between hepatotoxicity of halothane and sevoflurane and altered hepatic calcium homeostasis in enzyme-induced hypoxic rats.
METHODS: Forty-eight rats were pretreated with phen-obarbital and randomly divided into six groups (eight in each group) and exposed to O2/ N2/1.2 MAC anesthetics for 1 h: normal control (NC), 21% O2/79% N2; hypoxic control (HC), 14% O2/86%N2; normal sevoflurane (NS), 21% O2/ N2/1.2MAC sevoflurane; hypoxic sevoflurane (HS), 14% O2/ N2/1.2MAC sevoflurane; normal halothane (NH)21%O2/79%N2/1.2MAC halothane; hypoxic halothane (HH), 14% O2/N2/1.2MAC halothane. Liver specimens and blood were taken 24 h after exposure to calcium and determined by EDX microanalysis.
RESULTS: The liver of all rats given halothane (14% O2) had extensive centrilobular necrosis and denaturation. Morphologic damage was accompanied with an increase in serum glutamic pyruvic transminase. In groups NH and HH, more calcium was precipitated in cytoplasm and mitochondria.
CONCLUSION: These results suggest that halothane increases cytosolic Ca2+ concentration in hepatocytes. Elevation in Ca2+ concentration is implicated in the mechanism of halothane-induced hepatotoxicity. sevoflurane is less effective in affecting hepatic calcium homeostasis than halothane.
- Citation: Yu WF, Yang LQ, Zhou MT, Liu ZQ, Li Q. Ca2+ cytochemical changes of hepatotoxicity caused by halothane and sevoflurane in enzyme-induced hypoxic rats. World J Gastroenterol 2005; 11(32): 5025-5028
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/5025.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.5025
It is now recognized that two types of halothane-induced hepatic dysfunction exist[1,2]. A mild sub-clinical form manifested by abnormal biochemical indices of hepatic function can be caused by toxic products of halothane metabolism, possibly determined by genetic factors, or by hepatic hypoxia, resulting from an imbalance between hepatic oxygen supply and demand. A much rarer, fulminant form may occur with severe necrosis, which may prove fatal[3]. It is probable that this form results from an immune reaction: an oxidative metabolite binds covalently to liver proteins, producing a hypten, which in turn provokes immune reaction and formation of circulating antibody.
A major question being addressed in hepato-cellular injury is whether a unifying mechanism exists involving a loss of regulation of cellular Ca2+ levels. In this regard, alteration of Ca2+ homeostasis plays a major role in cell injury induced by a diversity of situations such as chemical intoxication and abnormal physiological states such as ischemia[4,5].
Animal studies have provided evidence supporting a role for altered calcium fluxes in the mechanism of halothane-induced liver injury. In guinea pigs, hepatic calcium content increases significantly 24 h after exposure to halothane. Subsequent changes in liver calcium are proportional to the severity of liver necrosis, as determined morphologically[6]. More recently it was shown that halothane, enflurane, and isoflurane stimulated dose-dependent release of radiolabeled calcium from internal calcium stores in isolated rat hepa-tocytes[7]. Further evidence in support of the carcinogenic hypothesis of cell injury is offered by studies, in which the administration of a calcium channel blocker reduces hepatic necrosis in animals exposed to hepatotoxic agents, including halothane[8]. For a better understanding of the mechanism of liver injury, attributing to halothane and sevoflurane hepatotoxicity, we used cytochemical methods to evaluate the changes of intracellular calcium and corresponding hepatic histopathological changes in enzyme-induced hypoxic rats.
The protocol was approved by the institutional Animal Care and Use committee. Adult male Sprague-Dawley rats weighing 150-160 g were obtained from the Animal Center of the Second Military Medical University and maintained in a 12 h dark-light cycle. The animals had free access to water and diet of Wayne rodent food. To induce the hepatic microsomal drug-metabolizing enzymes, 48 animals were given phenobarbital (1 mg/mL) in their drinking water for 10 d prior to any experiment[9].
For exposure to halothane and sevoflurane, animals were placed into 35 L plexiglass cages (g per cage). These animals were randomly divided into six groups and anesthetized for 1 h with O2/N2/1.2MAC anesthetic agents according to the following schedule: NC group was given 21% O2/79% N2; HC group 14% O2/86% N2; NNS group 21% O2/79% N2/1.2MAC sevoflurane; HS group 14% O2/86% N2/1.2MAC sevoflurane; NH group 21% O2/79% N2/1.2MAC halothane; HH group 14% O2/86% N2/1.2MAC halothane. Nitrogen and oxygen were delivered to the chamber by Dräger anesthetic machine at a flow rate of 4 L/min. The concentrations of O2/CO2, halothane, and sevoflurane in the chamber were monitored with a calibrated Capnormac Ultima.
After anesthesia or appropriate exposure, the animals were sent back into their metal cages and killed by decapitation, 24 h after anesthesia. Blood from the trunks was collected into dried beakers, and livers were rapidly removed and placed in chilled petridishes. Serum was separated from clotted blood, and assayed for ALT by automated methods in the Department of Clinical Chemistry. For histological examination , liver samples were collected into 10% PBS, fixed and mounted on paraffin blocks. Tissue sections were stained with hematoxylin and eosin, Gomori trichome and a reticulin stain. Coded liver sections were examined without knowledge of the experimental details. The necrosis and denaturation of the slides of each section were quantitatively estimated as previously described[10].
A portion of the right anterior lobe was cut into 0.5 mm blocks. The specimens were treated in cold fixative consisting of 25% glutaraldehyde in 0.9 mol/L potassium oxalate adjusted to pH 7.4 with 1 mol/L potassium hydroxide. Sucrose was added to 1.4% final concentration. Fixation was done for 4 h at 4 °C. The specimens were subsequently kept in a cold mixture of 1% osmium tetroxide and 2% potassium pyroantimonate for 2 h, followed by osmium tetroxide and 1% potassium ferrocyanide for 1 h. Then, the specimens were rinsed for 15 min with distilled water adjusted to pH 10 with 1 mol/L potassium hydroxide, dehydrated in cold ethanol series, and routinely embedded in Epon-812 or Spur.
The 100 nm thick sections for calcium cytochemistry, were left unstained and coated with carbon films in a vacuum evaporator. EDX microanalysis was performed under an analytical electron microscope (Hitachi-800) equipped with an energy-dispersive X-ray detecting system ( EDAX, type 9 100/60). The acceleration voltage was 100 KV and the probe current was for 100 s and evaluation of the energy-dispersive X-ray spectra was performed by a computer program[11].
Data were expressed as mean±SE and analyzed by analysis of variance. Means were compared with Fisher’s least significant difference test. P<0.05 was considered statistically significant.
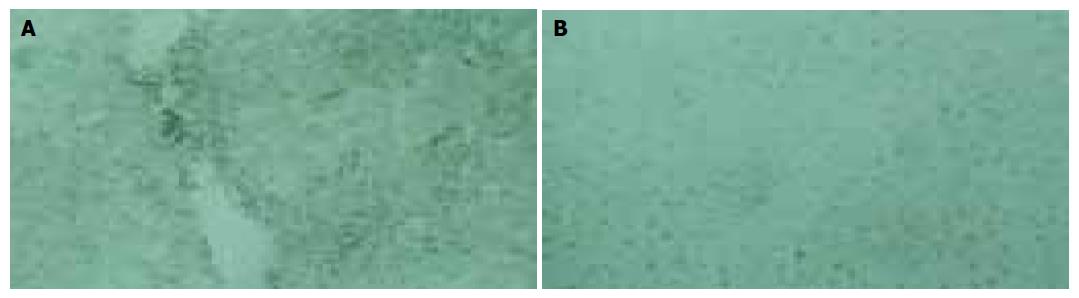
Under conditions of hypoxia and induction of the microsomal enzymes, halothane anesthesia produced extensive hepatic injury. Within 24 h after exposure to halothane at 14% O2, all the rats had many areas of hepatic necrosis radiating from the central veins. The necrosis and denaturation in HH group increased significantly as compared to the clusters of lymphocytes, histocytes and neutrophils, and often encircled by a layer of swollen hepatocytes containing single large vacuoles, strands of degenerating cytoplasm, and eccentric, intact pyknotic nuclei. Morphologic damage was accompanied with an increase in serum glutamic pyruvic transminase (P<0.01, Table 1). No statistically significant histologic change was found in the following variables: normal control, hypoxic control, halothane anesthesia at 21% O2, sevoflurane exposure at 21% O2 (Figure 1).
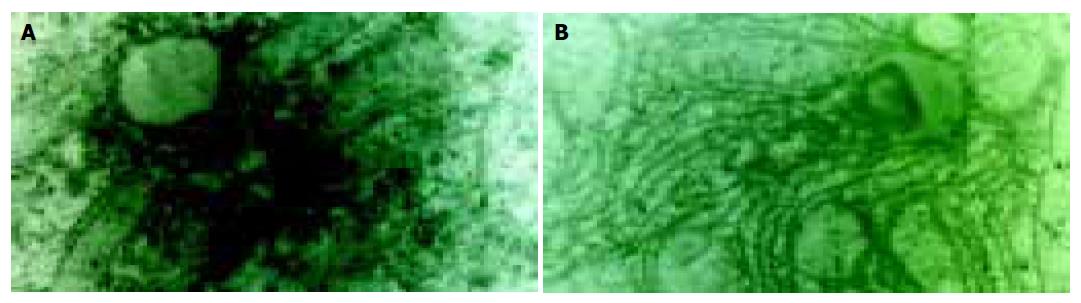
In NC and NS groups, calcium precipitation was located in nuclei with mitochondria and cytoplasm as fine particles. In HC and HS groups, intracellular calcium increased slightly. But after exposure to 21% O2/ N2/1.2MAC halothane or 14% O2/ N2/1.2MAC halothane, more and more calcium was precipitated in calcified cytoplasm and mitochondria. In HH group, a large amount of calcium deposition was found in cytoplasm and mitochondria (Figure 2).
Qualitative analyses were performed in nuclei, mitochondria and cytoplasm. The characteristic emission of calcium (Kal) was observed. Neither sodium nor potassium was present. Semi-quantitative analyses were performed in mitochondria and cytoplasm. The calcium emission analyses are shown in Table 2. The amount of cellular calcium increased in HH group (P<0.01) and there was a positive linear correlation between the calcium in mitochondria and cytoplasm.
The principle of calcium cytochemical technique is to use potassium pyroantimenate to deposit intracellular cations and can contribute to the understanding of cellular cation redistribution resulting from physiologic and pathologic stimuli. Because the precipitation by potassium pyroantimonate of cations is nonspecific, careful choice of reaction conditions for calcium cytochemistry is very important, and it must be done in conjunction with analytical techniques such as X-ray analysis to ascertain whether other cations are deposited[12]. When tissue is first fixed with glutaraldehyde and potassium pyroantimonate at low temperature (4 °C), better results can be acquired. Thus, the precipitation of calcium with cytochemical methods in combination with EDX microanalysis is valuable in investigating the mechanisms of hepatocellular injuries. Stereological methods provide the means of efficiently producing quantitative data on the internal structure of organs, tissues, and cells. These methods can easily be applied to cytological work at the light or electron microscopy level of resolution[13]. Although particular caution is indicated in avoiding systematic errors which may result from inadequate preparation, section thickness, etc, the results are generally very reliable.
Cytosolic Ca2+ concentration in hepatocytes may increase under hypoxic condition, which might be due to the changes of membrane functions, such as Ca2+- ATPase activity, Na+-Ca2+exchange system[14]. But cellular and mitochondrial calcium did not significantly increase in HC group. The causes are follows. (1) The degree of hypoxia (14% O2) is not severe; (2) Hypoxia differs from ischemia, and the substances synthetizing ATP do not exhaust; (3) Ca2+-ATPase activity may partly recover 24 h after hypoxic exposure for 1 h.
It has been proposed that hepatic damage occurs secondary to the disruption of mechanism, which maintains cellular calcium homeostasis. A retrospective study showed that there is evidence that halothane can elevate cytosolic free Ca2+ by releasing calcium from internal calcium stores and uptaking calcium from extracellular medium[15]. Recent work[16] has demonstrated that loss of sarcoplasmic reticulum’s Ca2+-ATPase activity by oxidizing agents, results direct oxidation of thiol groups on ATPase, but not lipid peroxidation. Halothane is also an oxidizing agent, but its mechanism underlying the increase of cytosolic Ca2+ is not clear. Sevoflurane, an inhalation anesthetic agent, undergoes considerable less metabolism and less disturbed Ca2+ homeostasis than halothane, which may be relevant to its lesser hepatotoxicity[17].
Cell injury due to loss of Ca2+ homeostasis correlates with blebbing of plasma membranes involving cytoskeletal proteins, Ca2+ ions and Ca2+dependent proteases[18]. The cytoskeletal protein is therefore expected to be a target for cytoplasmic calcium ions that promote changes in cell shape. Most of the known cytoskeletal receptors for calcium are associated with the actin filament system and these may be important in regulating many types of cell motility, including locomotion, phagocytosis, and secretion. Cytosolic free Ca2+ plays an increasingly important and fundamental role in the control of membrane permeability and cellular response to stimulation. Channel of discrete unitary conductance and selectivity activated by increased cytosolic free Ca2+ are responsible for K+ efflux transfer and Na+ influx, respectively. The elevation of mitochondrial Ca2+ influences mitochondrial respiration by changing activities of three matrix enzymes, pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase and isocitrate dehydrogenase. Ca2+ activates proteases and endonucleases. Ca2+ enhances formation of active oxygen species, etc.
Sugimura et al[19] found that calcium channel blocking agent inhibits the production of radical intermediates during metabolism of halothane. These results suggest that calcium can activate the metabolism of halothane. Thus, we consider that the viscous circle of peroxidation activated by radical intermediates and elevation of cytosolic calcium may be the basis of halothane -induced hepatotoxicity under hypoxic internal environment.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Bond GR. Hepatitis, rash and eosinophilia following trichloroethylene exposure: a case report and speculation on mechanistic similarity to halothane induced hepatitis. J Toxicol Clin Toxicol. 1996;34:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Kenna JG. The molecular basis of halothane-induced hepatitis. Biochem Soc Trans. 1991;19:191-195. [PubMed] |
| 3. | El-Bassiouni EA, Abo-Ollo MM, Helmy MH, Ismail S, Ramadan MI. Changes in the defense against free radicals in the liver and plasma of the dog during hypoxia and/or halothane anaesthesia. Toxicology. 1998;128:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Iaizzo PA, Seewald MJ, Olsen R, Wedel DJ, Chapman DE, Berggren M, Eichinger HM, Powis G. Enhanced mobilization of intracellular Ca2+ induced by halothane in hepatocytes isolated from swine susceptible to malignant hyperthermia. Anesthesiology. 1991;74:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Frost L, Prendergast D, Farrell G. Halothane hepatitis: damage to peripheral blood mononuclear cells produced by electrophilic drug metabolites is Ca(2+)-dependent. J Gastroenterol Hepatol. 1989;4:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Frost L, Mahoney J, Field J, Farrell GC. Impaired bile flow and disordered hepatic calcium homeostasis are early features of halothane-induced liver injury in guinea pigs. Hepatology. 1996;23:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Araki M, Inaba H, Kon S, Imai M, Mizuguchi T. Effects of volatile anesthetics on the calcium ionophore A23187-mediated alterations in hepatic flow and metabolism in the perfused liver in fasted rats. Acta Anaesthesiol Scand. 1997;41:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Satorres J, Pérez-Mateo M, Mayol MJ, Esteban A, Graells ML. Protective effect of diltiazem against acetaminophen hepatotoxicity in mice. Liver. 1995;15:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Yamazoe Y, Shimada M, Murayama N, Yamauchi K, Kato R. Alteration of hepatic drug metabolizing activities and contents of cytochrome P-450 isozymes by neonatal monosodium glutamate treatment. Biochem Pharmacol. 1988;37:1687-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Barańska W, Baran W, Skopiński P, Ziemba H. Morphometric analysis of satellite cells in rat skeletal muscles: soleus and extensor digitorum longus. Acta Anat (Basel). 1997;160:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Jonas L, Fulda G, Kröning G, Merkord J, Nizze H. Electron microscopic detection of tin accumulation in biliopancreatic concrements after induction of chronic pancreatitis in rats by di-n-butyltin dichloride. Ultrastruct Pathol. 2002;26:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Musetti R, Favali MA. Cytochemical localization of calcium and X-ray microanalysis of Catharanthus roseus L. infected with phytoplasmas. Micron. 2003;34:387-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Makita T, Itagaki S, Ohokawa T. X-ray microanalysis and ultrastructural localization of cisplatin in liver and kidney of the rat. Jpn J Cancer Res. 1985;76:895-901. [PubMed] |
| 14. | Chang YJ, Chang KJ. Calcium concentration in rat liver mitochondria during anoxic incubation. J Formos Med Assoc. 2002;101:136-143. [PubMed] |
| 15. | Byrne AM, Lemasters JJ, Nieminen AL. Contribution of increased mitochondrial free Ca2+ to the mitochondrial permeability transition induced by tert-butylhydroperoxide in rat hepatocytes. Hepatology. 1999;29:1523-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Favero TG, Webb J, Papiez M, Fisher E, Trippichio RJ, Broide M, Abramson JJ. Hypochlorous acid modifies calcium release channel function from skeletal muscle sarcoplasmic reticulum. J Appl Physiol (1985). 2003;94:1387-1394. [PubMed] |
| 17. | Johansson JS, Zou H. Partitioning of four modern volatile general anesthetics into solvents that model buried amino acid side-chains. Biophys Chem. 1999;79:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Volbracht C, Leist M, Nicotera P. ATP controls neuronal apoptosis triggered by microtubule breakdown or potassium deprivation. Mol Med. 1999;5:477-489. [PubMed] |
| 19. | Sugimura M, Kitayama S, Morita K, Irifune M, Takarada T, Kawahara M, Dohi T. Effects of volatile and intravenous anesthetics on the uptake of GABA, glutamate and dopamine by their transporters heterologously expressed in COS cells and in rat brain synaptosomes. Toxicol Lett. 2001;123:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |