Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.5022
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: August 28, 2005
AIM: To observe the expressions of early growth response factor-1 (Egr-1) and tissue factor (TF) in rats with cerulein-induced acute pancreatitis and to explore its significance.
METHODS: A large dose of cerulein was used to create the experimental acute pancreatitis model in rats. The changes of Egr-1 mRNA and protein in rats were observed during 30 min to 4 h after the treatment and immunohistochemical method was used to observe the localized expression of Egr-1 in tissues. In addition to the mRNA expression of Egr-1 target gene, TF was also observed. A blank control group, and a bombesin-administered group were used for comparison.
RESULTS: After the stimulation of a large dose of cerulein, the rats showed typical inflammatory changes of acute pancreatitis. Thirty minutes after the stimulation, the mRNA expression of Egr-1 in the pancreatic tissue reached its peak and then declined, while the expression of Egr-1 protein reached its peak 2 h after the stimulation. Histologically, 2 h after the stimulation, almost all pancreatic acinar cells had the expression of Egr-1 protein, which was focused in the nuclei. The mRNA expression of TF occurred 1 h after the stimulation and gradually increased within 4 h. However, a large dose of bombesin only stimulated the pancreatic tissue to produce a little mRNA expression of Egr-1 and no mRNA expression of Egr-1 protein and TF.
CONCLUSION: Egr-1 as a pro-inflammatory transcription factor may play an important role in the pathogenesis of acute pancreatitis by modulating the expression of TF.
- Citation: Gong LB, He L, Liu Y, Chen XQ, Jiang B. Expression of early growth response factor-1 in rats with cerulein-induced acute pancreatitis and its significance. World J Gastroenterol 2005; 11(32): 5022-5024
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/5022.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.5022
Acute pancreatitis is a common disease with its severe case having a mortality of up to 25-40%, while its pathogenesis still remains unclear[1,2]. The activation of pancreatic enzyme in acinar cells and its digestion of pancreatic tissues have been generally regarded as its trigger so far. However, recent studies on transcription factor, nuclear factor-κB (NFκB) and other cytokines showed that inflammatory cytokines play an important role in the pathogenesis of acute pancreatitis due to their inflammation-inducing properties[3-7]. Early growth response factor-1 (Egr-1), also known as nerve growth factor-induced protein A, Krox-24 or Zif268, is a transcription factor -NFκB[8]. A recent study showed that Egr-1 may be an initial factor of inflammation[9]. In our experiment, we studied the expression of Egr-1 in rats with cerulein-induced acute pancreatitis to understand its role in the pathogenesis of acute pancreatitis.
Following the procedures for construction of animal models[3,6], cerulein was dissolved in normal saline. Then cerulein solution [20 μg/(kg·h)] was injected into abdominal cavity of male Wistar rats. After 4 h of the stimulation, a large dose of sodium pentobarbital was injected into the abdominal cavity of the rats, followed by incision of the thorax cavity to collect blood by a syringe needle; besides, a little lung tissue was harvested. Subsequently, an incision was made to open the abdominal cavity to expose the pancreatic tissue and a piece of pancreatic tissue was harvested and put into the pre-cooled neutral PBS (pH 7.4) with its fat removed. Pancreatic tissue was fixed in neutral 10% paraformaldehyde and cooled in liquid nitrogen and preserved at -70 °C. The blood was centrifuged for blood serum, which was then preserved at -70 °C.
The acute pancreatitis indexes included serum amylase, tissue myeloperoxidase (MPO) and percentage water content. The test procedures are described in our previous study[6].
According to the method described in our previous study[6], the cooled pancreatic tissue was homogenized and centrifuged with its supernatant for protein quantification. About 25 µg protein was added into each hole for PAGE-SDS gel electrophoresis, the product of which was then transferred to PVDF film for incubation with an antibody at -4 °C overnight. The immunoblotting protein band was detected using emission chemiluminescence kit. The film was scanned and saved as a digital image (Figure 1). The antibody used in our study was from Santa Cruz Company.
The fresh pancreatic tissue was put into TRIzol (Invitrogen) for homogenate and then chloroform and isopropanol were added, followed by centrifugation of supernatant to obtain DNA by ethanol precipitation. The obtained DNA was then digested with DNA enzyme I and purified by RNA purification (Qiagen). Through qualification of RNA, RT kit (Promega) was used for transcription to obtain cDNA, which was then amplified by routine PCR. SYBR (Bio-Rad) dye was added and 27-32 cycles were amplified, the relative production was detected with quantitative PCR system. The result was shown in a ratio of the production to APRO. The primers used were Egr-1 (GenBank NM_012551) of sense: 5’CCGTATGCTTGCCCTGTTGAGTC3’ and antisense: 5’CCGTTGAGGTGCTGAAGGAGTTG3’ with PCR product of 548 bp and tissue factor (TF, GenBank U07619) of sense: 5’GTTCAGATAAGCGATAGA3’ and antisense: 5’TGCGAAGAAGCAGTAG3’ with a PCR product of 482 bp as control, rat acidic ribosomal phosphoprotein (GenBank X15096) of sense: 5’CGC-GGGAAGGCTGTGGTG3’ and antisense: 5’GGGC-CTGCTCTGTGATGTC3’ with a PCR product of 492 bp as control.
The tissue was processed routinely, dehydrated and paraffin-embedded, cut into 5-µm-thick sections and stained with hematoxylin and eosin. Then, the histological and morphological changes were observed under an optical microscope. During immunohistochemistry, the tissue sections were routinely dewaxed and liquefied and rabbit Egr-1 antibody was added. Then, Egr-1 was detected.
The data were expressed as mean±SD and analyzed with SPSS 1.0 software package.
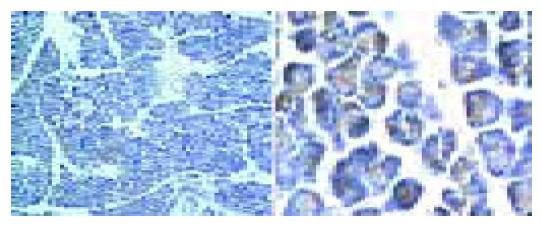
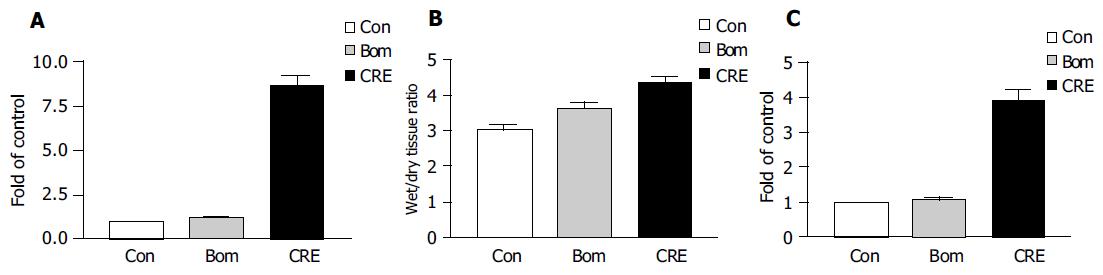
Large dose of cerulein-induced obvious acute pancreatitis and the pancreatic tissue had the manifestations of acute abscess, widened tissue interspace and obvious, swollen pancreatic acinar cells (Figure 1). The indexes for diagnosis of acute pancreatitis, including blood serum amylase, tissue MPO, and percentage of water content, significantly increased (Figure 2). Similar to the previous report[10], bombesin failed to induce typical experimental acute pancreatitis. Large dose of bombesin induced only tissue edema, which was relatively milder than that induced by cerulein, amylase, and MPO increased with no statistical significance (Figure 2).
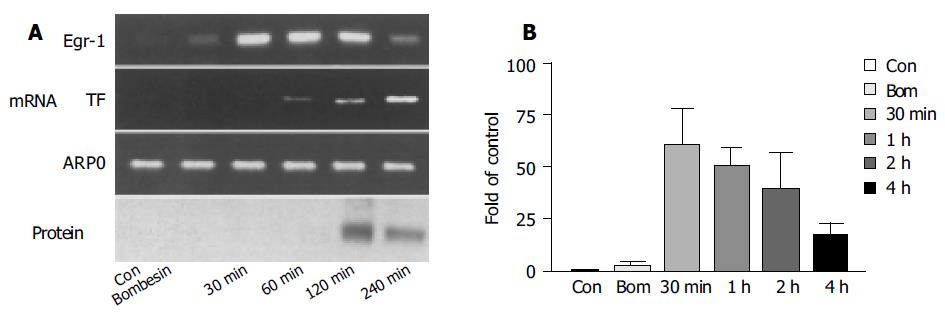
The expression of Egr-1 mRNA reached its peak 30 min after the injection of cerulein, which was 60 times higher than that in the control (Figure 3B). However, it significantly declined after 4 h. Correspondingly, the expression of Egr-1 protein occurred 2 h after injection and obviously declined after 4 h. Egr-1 protein expression occurred 2 h after the stimulation, and almost all acinar cells had the expression of Egr-1 protein, while the expression was locally stronger and the expression of Egr-1 was found in nuclei. The expression of TF was later than that of Egr-1. Obvious expression of TF mRNA was seen 1 h after the stimulation and peaked in 2-4 h (Figure 3A).
Recent studies have shown that Egr-1, as one of the transcription factors during the early stage of inflammation, can regulate the expression of multiple inflammatory factors, such as tumor necrosis factor, interleukin-2, transforming growth factor-β1, intracellular adhesion molecule-1 (ICAM-1), and TF, and eradication of Egr-1 can protect lungs from injuries induced by ischemia and decrease the expression of tissue IL-1β, IL-6, ICAM-1, MCP-1, TF, and PAI[9]. However, the expression and function of Egr-1 in acute pancreatitis remain unclear.
In our study, during the early stage of acute pancreatitis induced by cerulein, Egr-1 demonstrated high expression in mRNA and protein. Its protein expression occurred 1 h after stimulation, peaked at 2 h, and lasted for at least 4 h. Egr-1 was found in nuclei 2 h after stimulation in our experiment, indicating that Egr-1 may play an important regulatory role during the early stage of acute pancreatitis. We also found that the expression of Egr-1 in acinar cells was locally distinct, which may be related to the property of Egr-1 that it is extremely easy to attenuate. Being different from cerulein, bombesin could not induce typical acute pancreatitis[10] and did not increase blood serum amylase and tissue MPO level although bombesin could also induce mild edema of pancreatic tissue. Correspondingly, bombesin induced only trace expression of Egr-1 mRNA, but expression of protein could not be detected. The result further indicates, that the expression of Egr-1 plays a role in the pathogenesis of acute pancreatitis.
Previous studies have shown that during the initial and developmental process of acute pancreatitis, ischemia-reperfusion plays an important role[11] and TF is an initial factor for coagulation, which can result in decrease of the tissue blood flow and ischemia[8,9]. It was reported that TF is an important targeting factor of Egr-1, whose expression is modulated by Egr-1[9]. Our study indicated that during the early stage of acute pancreatitis, TF also had high expression. However, compared to Egr-1, its expression was 1-2 h later. Therefore, we believe that during acute pancreatitis, Egr-1 may regulate the expression of TF and trigger coagulation to induce ischemia of pancreatic tissue, thus aggravating the disease. Nevertheless, the detailed mechanism of TF during acute pancreatitis needs further studies.
In conclusion, Egr-1, as a pro-inflammatory transcription factor that may play an important role in the pathogenesis of acute pancreatitis.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Steer ML. Pathobiology of experimental acute pancreatitis. Yale J Biol Med. 1992;65:421-430; discussion 437-440. [PubMed] |
| 2. | Lerch MM, Gorelick FS. Early trypsinogen activation in acute pancreatitis. Med Clin North Am. 2000;84:549-563, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402-G1414. [PubMed] |
| 4. | Grady T, Liang P, Ernst SA, Logsdon CD. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;113:1966-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 159] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Frossard JL, Pastor CM, Hadengue A. Effect of hyperthermia on NF-kappaB binding activity in cerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1157-G1162. [PubMed] |
| 6. | Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Gashler A, Sukhatme VP. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol. 1995;50:191-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 486] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 9. | Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 387] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Powers RE, Grady T, Orchard JL, Gilrane TB. Different effects of hyperstimulation by similar classes of secretagogues on the exocrine pancreas. Pancreas. 1993;8:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Gullo L, Cavicchi L, Tomassetti P, Spagnolo C, Freyrie A, D'Addato M. Effects of ischemia on the human pancreas. Gastroenterology. 1996;111:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |