Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.5010
Revised: December 20, 2004
Accepted: December 23, 2004
Published online: August 28, 2005
AIM: To compare the effects of neurolytic celiac plexus block (NCPB) and videothoracoscopic splanchnicectomy (VSPL) on pain and quality of life of chronic pancreatitis (CP) patients.
METHODS: Forty-eight small duct CP patients were treated invasively with NCPB (n = 30) or VSPL (n = 18) in two non-randomized, prospective, case-controlled protocols due to chronic pain syndrome, and compared to a control group who were treated conservatively (n = 32). Visual analog scales were used to assess pain and opioid consumption rate was evaluated. In addition, the quality of life was measured using QLQ C-30 for NCPB and FACIT for VSPL. Although both questionnaires covered similar problems, they could not be compared directly one with another. Therefore, the studies were compared by meta-analysis methodology.
RESULTS: Both procedures resulted in a significant positive effect on pain of CP patients. Opioids were withdrawn totally in 47.0% of NCPB and 36.4% of VSPL patients, and reduced in 53.0% and 45.4% of the respective patient groups. No reduction in opioid usage was observed in the control group. In addition, fatigue and emotional well-being showed improvements. Finally, NCPB demonstrated stronger positive effects on social support, which might possibly be attributed to earlier presentation of patients treated with NCPB.
CONCLUSION: Both invasive pain treatment methods are effective in CP patients with chronic pain.
- Citation: Basinski A, Stefaniak T, Vingerhoets A, Makarewicz W, Kaska L, Stanek A, Lachinski AJ, Sledzinski Z. Effect of NCPB and VSPL on pain and quality of life in chronic pancreatitis patients. World J Gastroenterol 2005; 11(32): 5010-5014
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/5010.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.5010
Pain is the most significant, frequent and difficult to treat symptom of chronic pancreatitis (CP). The pain is severe, persistent, excruciating and unbearable[1-4]. It is often localized in the epigastrium, radiates to the left or more frequently to the right side, or to the lower part of the thoracic vertebral column or to the right scapula[2-5]. The pain is often accompa-nied with nausea and vomiting. The underlying pathop-hysiological mechanisms of pain are still unknown.
Typical CP pain is associated with increased pancreatic duct pressure and caused by stimulation of specific nociceptors or sensing receptors[3]. Currently, the most significant reason of pain in CP is considered to be the pressure of pancreatic fluid increased intraductally and within the pancreatic tissue. This may result in impaired outflow of pancreatic fluid and may also present as pancreatic pseudocysts (in cases of pancreatic duct rupture)[1]. It can also be influenced by self-digestion of pancreatic ducts due to progressing inflammation and by inflammation of nerves involved in transfer of stimuli from the pancreas (pancreatitis associated neuritis)[6].
Afferent fibers from the celiac region may enter the spinal cord at a different level from which the respective efferent fibers originate. Fibers of these bunches enter the gray matter and terminate within layers I and IV[9]. There is no morphological or functional evidence for the existence of an indirect celiac projection in the layer II, contrary to the somatic projection found extensively in layers I and II[7-9]. Thus, the distribution of celiac and somatic stimuli within the spinal cord is different. Nevertheless, the fact that visceral pain is sometimes associated with somatic structures and convergence of the stimuli from organs and somatic structures into sensory neurons may further initiate a sensation of somatic pain[10].
It is generally acknowledged that the quality of life of CP patients significantly decreases due to severe and constant pain[1,4]. Various methods, including invasive techniques, have been proposed to treat pain[11]. The aim of the present study was to compare the effect of two most frequently applied methods of treatment, NCPB and videothoracoscopic splanchnicectomy (VSPL) on pain, opioid consumption rate and quality of life of CP patients.
Two groups of small-duct CP patients were treated in two separate prospective, case-controlled studies with NCPB (n = 30) or VSPL (n = 18). In addition, a control group (n = 32) consisting of CP patients with chronic pain syndrome managed conservatively took part in this study.
The patients chose the procedure according to their needs (consumeric approach – orientation towards the needs of patients).
All the procedures were performed by surgeons experienced in videoscopic surgery.
The demographic data of the three groups are presented in Table 1. No patient had structural lesions: pancreatic duct stricture, pseudocyst, or distal common bile duct stricture amenable to either endoscopic or surgical treatment. The inclusion criteria were a CP diagnosed by CT scan and endoscopic retrograde cholangiopancreatography, persistent pain for at least 3 mo, scoring at least 66.7% on the pain visual analog scale. Patients with pancreatic inflammatory tumors or pseudocysts were excluded from the study.
| Protocol 1 NCPB (n = 30) | Protocol 2 VSPL (n = 18) | Control (n = 32) | |
| Age (yr) | 49.9±7.8 | 47.3±5.0 | 52.3±6.6 |
| Sex (M:F ratio) | 3.01 | 3.51 | 3.34 |
| Etiology (alcohol vs other ratio) | 6.5 | 8 | 7 |
| Period from the symptomatic onset of the disease | 6.2±2.2 | 11.5±4.3 | 7.1±3.0 |
Pain was measured using visual analog scales and quality of life of CP patients was assessed by well-validated EORTC, quality of life questionnaire QLQ C-30 and functional asses-sment of chronic illness therapy (FACIT). The control group completed both health-related quality of life measurements. The patients were assessed 2 d before the treatment, then followed up and reassessed after 2nd and 8th wk.
The patients were fixed in the prone position with a slight bending forward. The lower margin of the 12th rib was marked on both sides of the body. After the superficial anesthesia with 1% lignocaine, the 20-G needle (10-12 cm) pierced into the point located 5-7 cm laterally from the midline on both sides just under the lower margin of the 12th rib, at the angle of 30-45 towards the trunk of L1 and Th12 vertebrae or the space between L1 and Th12 vertebrae. The canal of the needle was then additionally anesthetized with further 6-10 mL of 1% lignocaine. The needle was pierced into until the resistance of the bone was met. If this resistance was met at the level of 3-5 cm under the skin, it was assumed that the needle reached the vertebra and the needle was withdrawn and re-introduced in order to bypass above or below the vertebra towards its lateral surface. The surface was achieved at the depth of 6-8 cm. Then, the needle was rotated 90 towards the vertebra and re-introduced with a continuous decrease of the angle of the introduction, aiming to slide on the lateral surface of the vertebra. When the needle bypassed the vertebra by 1-2 cm, and after reassuring that it was not located in the aorta or other major vessel, 20-30 mL of 50% ethanol with 5 mL of 1% lignocaine was injected into both sides.
Because unilateral (preferably left-sided) splanchnicectomy was reported to be adequate in control of the intractable pancreatic pain[12], all patients were given a left-sided intervention. General anesthesia was administered with single-bronchus intubation in every case. The patient was placed in the right lateral decubitus position with the left arm elevated at a 90° angle, and fixed with support-arms and bandages, and the table was then tilted 30° anteriorly in the longitudinal axis (thus, after induction of a pneumothorax, the lung would “fall away” from the costovertebral gutter). After desufflation of the lung, two trocars were inserted into the thorax: one (a 5-mm trocar) was placed in the fifth intercostal space in a medial axillary line for the camera, and the second (a 5-mm trocar) was placed in the seventh intercostal space in an anterior axillary line for the instruments. After identification of the splanchnic nerve, situated above the aorta on the left or above the azygos vein on the right, the parietal pleura was incised and the nerve together with its minor connecting branches, was prepared to a distance of 5-8 cm and then excised. After insufflation of the lung, the trocars were removed, a single chest tube was placed, and the wounds were closed according to surgical standards.
This study consisted of two separate, prospective, non-randomized, case-controlled experiments (NCPB vs control and VSPL vs control). By consequence, the statistical techniques applied in meta-analysis were employed, fixed-effects and effect sizes were calculated as the difference between the outcome means of the groups divided by the pooled standard deviations. An overall weighed effect size was obtained and presented with 95% confidence interval (95%CI). Results were tested for homogeneity using the Breslow-Day tests.
Parametric data were tested with t test, ANOVA, and non-parametric estimations were conducted with U Mann-Whitney and χ2 tests. All analyses were performed using Statistica 6.0 software licensed to Medical University of Gdansk.
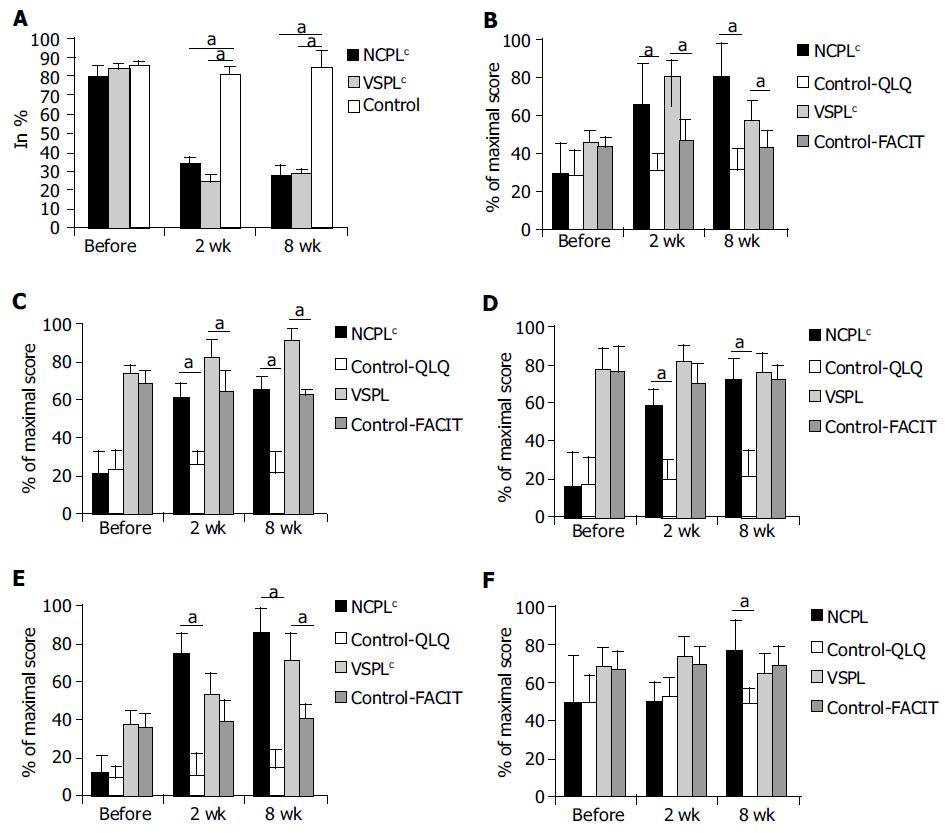
The raw data and univariate statistics for both protocols are shown in Figure 1.
In both groups of CP patients, the therapeutic intervention was beneficial (Table 2).
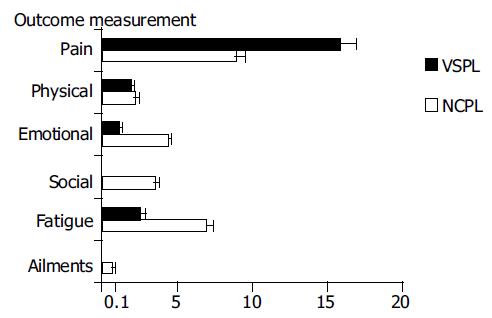
| Therapy | Group size | Parameter | Mean effect | 95% CI (min-max) |
| VSPL | 18 | Paina | 15.82 | (14.68-16.96) |
| NCPL | 30 | 8.89 | (8.30-9.48) | |
| VSPL | 18 | Physical well-beinga | 1.81 | (1.57-2.06) |
| NCPL | 30 | 2.19 | (1.96-2.42) | |
| VSPL | 18 | Emotional well-beinga | 1.12 | (0.91-1.34) |
| NCPL | 30 | 4.4 | (4.07-4.73) | |
| VSPL | 18 | Social supporta | 0.08 | (-0.11-0.29) |
| NCPL | 30 | 3.55 | (3.27-3.84) | |
| VSPL | 18 | Fatigue | 2.52 | (2.25-2.79) |
| NCPL | 30 | 6.87 | (6.39-7.34) | |
| VSPL | 18 | Ailments typical for the illnessa | 0.05 | (-0.14-0.26) |
| NCPL | 30 | 0.64 | (0.45-0.83) |
The effects were significant and very strong, especially concerning pain, as indicated on VAS scale. In order to control for possible regression to the mean effect, the mean effect sizes were calculated by comparison of the results after 2nd and 8th wk, not only to the pre-treatment baseline, but also to the results of the control group treated conservatively and evaluated at the corresponding periods of follow-up.
Opioids were administered to 17 patients (56.7%) in the NCPB group and to 11 patients (61.1%) in the VSPL group. In the control group, 18 patients (56.2%) were treated with opioids and this number was not altered during the follow-up. The initial frequency of opioid treatment did not differ between the groups. The opioids were withdrawn totally after invasive treatment in 8 (47%) NCPB patients, and in 4 (36.4%) VSPL patients, and the dosage was reduced in another 9 (53%) and 5 (45.4%) patients, respectively (no statistically significant difference). No changes were observed throughout the whole period of follow-up. In two remaining opioid-using patients (18.2%) after VSPL, no long-term opioid reduction could be achieved (Figure 2).
There was no mortality and no major morbidity. Orthostatic hypotension was observed for 3 d in nine patients (30%) from NCPB group and in one patient (5.5%) from VSPL group. Diarrhea was resolved after symptomatic treatment for 14 d in four patients (13.3%) from the NCPB group. Intermittent intercostal pain was treated with paracetamol for 2 wk in four patients (22.2%) from VSPL group. In one of them (5.5%), intercostal nerve block had to be instituted. In one case, classic thoracotomy had to be performed due to massive adhesions (who was excluded from the study).
In the presented study, the effects of NCPB and VSPL on CP patients and controls were compared. As the methods for assessment of life quality differed in two protocols, the comparison was performed by meta-analytic approach. The reduction of pain was stronger in VSPL group than in NCPB group, but the effects in both groups were very potent.
The influence on physical well-being was slightly better in NCPB group, but did not differ statistically from the VSPL group. No improvement concerning ailments typical for the disease was noticed. This finding can be explained by the fact that the analgesic treatment cannot obviously affect the cause of the disease.
Interestingly, the effect on social support and emotional well-being was significantly stronger in NCPB group than in VSPL group. Moreover, the effect of VSPL on social support was weak, while it was very strong after neurolysis. This may be explained by the fact, that this procedure is the first available treatment for the patients in the NCPB group, whereas the VSPL patients have already tried some other treatments, thus having longer history of chronic pain syndrome. Therefore, the decision to receive an invasive treatment is more intensely approved by the NCPB group and the improvement resulting from this treatment is very likely more appreciated emotionally by the patients.
Since conservative treatment of CP chronic pain syndrome is not always effective and may be associated with significant morbidity of iatrogenic opioid addiction, both at physical (suppression of immune function, chronic constipation, drowsiness, and fatigue[13,14]) and psychological level (decrease in social support, depression, addiction behavior)[14], an attention has been paid to more invasive methods for pain control.
In the present study, the reduction of opioid consumption was significant in both groups, which gives an optimistic perspective for minimizing the adverse effects of this group of drugs, especially in the chronic treatment. Nevertheless, in two patients treated with opioids for more than a year, VSPL did not result in decrease of the opioid consumption or withdrawal. As early as 3 d after the procedure, the patients complained about persistent pain localized on the opposite side of the abdomen. The pain could only be alleviated by pethidine. As to date, no diagnostic or treatment guidelines for suspected iatrogenic opioid addiction are available, opioid analgesics were administered. It should be highlighted that administering opioids ought to be considered as the last resort and should only be applied, if any other method has failed. Since the life expectancy of CP patients is 10-20 years shorter than that of general population[15], the adverse effects of iatrogenic opioid addiction are very likely to occur in these patients. In contrast, the life expectancy of pancreatic cancer rarely exceeds 12 mo[16].
The pathogenesis of pain in CP still remains a mystery. The factors postulated in its genesis include the release of excessive oxygen-derived free radicals, tissue hypoxia and acidosis, inflammatory infiltration with influx of pain-transmitting substances into damaged nerve ends, and the development of pancreatic ductal and tissue fluid hypertension due to morphological changes of the pancreas[1-5]. Di Sebastiano et al[6] reported that the possible additional relevant pancreatic pain pathology factors are ischemia as well as inflammatory and autoimmune reactions. Toma et al[17] described that expression of the neurotrophin nerve growth factor increased during the course of experimental acute pancreatitis in rats. In human CP, neurotrophin gene expression correlates with the intensity of pain[18], so that pathogenesis could be speculated in acute phases of CP. Keith et al[19] proposed that neural and perineural alterations may be important in pain pathogenesis of CP, and concluded that pain severity correlates with duration of alcohol consumption, pancreatic calcification, and percentage of eosinophils in perineural inflammatory cell infiltrates, but not with duct dilatation.
Bockman et al[20] have provided evidence for an increase in both the number and diameter of pancreatic nerve fibers during the course of CP. In tissue specimens from patients suffering from CP, foci of chronic inflammatory cells are often found surrounding pancreatic nerves exhibiting a damaged perineurium and invasion by lymphocytes. These abnormalities might allow free access of inflammatory mediators or active pancreatic enzymes into nerves, generating and sustaining pain.
The pro-inflammatory cytokine, IL-8 is a well-known chemokine involved in leukocyte recruitment, and activation of IL-8 release generates hyperalgesia by stimulation of post-ganglionic sympathetic neurones. A significant increase in IL-8 mRNA has been reported in CP tissue samples[21]. Major sources of IL-8 are inflammatory cells present around enlarged nerves in the inflammatory foci. Thus, induction of IL-8 in immune cells might contribute to amplification of the inflammatory process in CP. The release of IL-8 from the remaining exocrine pancreatic parenchyma may indicate the intrinsic maintenance of the inflammatory response after the first damage to the pancreatic gland, thus sustaining progression and evolution of the disease[6].
It has been recently suggested that differential epidural anesthesia might be beneficial for differentiating cases susceptible to further sympathetic pain treatment from those who are resistant to it[22]. Moreover, we would add that the use of NCPB as a therapeutic and diagnostic procedure might be crucial for selecting patients for further VSPL treatment in case of the ailment recurrence. Thus, NCPB should be suggested as the first step of invasive analgetic therapy, as soon as the opioid treatment is needed. Vranken et al[23] have demonstrated that the efficacy of the second NCPB in CP is significantly lower than that of the first, and that the pain-free period is usually shorter. Therefore, when all the non-opioid methods of analgesia are exhausted, the second step would be left-sided VSPL. Due to rare but fairly significant complications following bilateral splanc-hnicectomy[24,25], we would favor unilateral or bilateral non-simultaneous VSPL in CP patients. Only after both-sided VSPLs have been performed and additional NCPB is also attempted, use of opioids is justified in CP patients.
It should also be stressed that the present analysis covers a relatively short period of follow-up. Moreover, the use of two different quality of life measurements may be perceived as another drawback of the present study. Therefore, future studies should include randomization, uniform outcome assessment, and longer follow-up assessment.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Pitchumoni CS. Pathogenesis and managenent of pain in chronic pancreatitis. World J Gastroenterol. 2000;6:490-496. [PubMed] |
| 2. | Witzigmann H, Max D, Uhlmann D, Geissler F, Schwarz R, Ludwig S, Lohmann T, Caca K, Keim V, Tannapfel A. Outcome after duodenum-preserving pancreatic head resection is improved compared with classic Whipple procedure in the treatment of chronic pancreatitis. Surgery. 2003;134:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Di Sebastiano P, Friess H, Di Mola FF, Innocenti P, Büchler MW. Mechanisms of pain in chronic pancreatitis. Ann Ital Chir. 2000;71:11-16. [PubMed] |
| 4. | Sohn TA, Campbell KA, Pitt HA, Sauter PK, Coleman JA, Lillemo KD, Yeo CJ, Cameron JL. Quality of life and long-term survival after surgery for chronic pancreatitis. J Gastrointest Surg. 2000;4:355-64; discussion 364-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Sakorafas GH, Anagnostopoulos G. Surgical management of chronic pancreatitis: current concepts and future perspectives. Int Surg. 2003;88:211-218. [PubMed] |
| 6. | Di Sebastiano P, di Mola FF, Bockman DE, Friess H, Büchler MW. Chronic pancreatitis: the perspective of pain generation by neuroimmune interaction. Gut. 2003;52:907-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Gohlke F, Janssen E, Leidel J, Heppelmann B, Eulert J. Histopathological findings in the proprioception of the shoulder joint. Orthopade. 1998;27:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | de Leon-Casasola OA. Critical evaluation of chemical neurolysis of the sympathetic axis for cancer pain. Cancer Control. 2000;7:142-148. [PubMed] |
| 9. | Cervero F, Connell LA. Fine afferent fibers from viscera do not terminate in the substantia gelatinosa of the thoracic spinal cord. Brain Res. 1984;294:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Jänig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 204] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Makarewicz W, Stefaniak T, Kossakowska M, Basiński A, Suchorzewski M, Stanek A, Gruca ZB. Quality of life improvement after videothoracoscopic splanchnicectomy in chronic pancreatitis patients: case control study. World J Surg. 2003;27:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, Mantilla CB, Warner DO. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 289] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Portnow JM, Strassman HD. Medically induced drug addiction. Int J Addict. 1985;20:605-611. [PubMed] |
| 15. | Strate T, Yekebas E, Knoefel WT, Bloechle C, Izbicki JR. Pathogenesis and the natural course of chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Liu SL, Friess H, Kleeff J, Ji ZL, Büchler MW. Surgical approaches for resection of pancreatic cancer: an overview. Hepatobiliary Pancreat Dis Int. 2002;1:118-125. [PubMed] |
| 17. | Toma H, Winston J, Micci MA, Shenoy M, Pasricha PJ. Nerve growth factor expression is up-regulated in the rat model of L-arginine-induced acute pancreatitis. Gastroenterology. 2000;119:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Friess H, Zhu ZW, di Mola FF, Kulli C, Graber HU, Andren-Sandberg A, Zimmermann A, Korc M, Reinshagen M, Büchler MW. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg. 1999;230:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Keith RG, Keshavjee SH, Kerenyi NR. Neuropathology of chronic pancreatitis in humans. Can J Surg. 1985;28:207-211. [PubMed] |
| 20. | Bockman DE, Buchler M, Malfertheiner P, Beger HG. Analysis of nerves in chronic pancreatitis. Gastroenterology. 1988;94:1459-1469. [PubMed] |
| 21. | Di Sebastiano P, di Mola FF, Di Febbo C, Baccante G, Porreca E, Innocenti P, Friess H, Büchler MW. Expression of interleukin 8 (IL-8) and substance P in human chronic pancreatitis. Gut. 2000;47:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Bradley EL, Bem J. Nerve blocks and neuroablative surgery for chronic pancreatitis. World J Surg. 2003;27:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Vranken JH, Zuurmond WW, de Lange JJ. Increasing the efficacy of a celiac plexus block in patients with severe pancreatic cancer pain. J Pain Symptom Manage. 2001;22:966-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Howard TJ, Swofford JB, Wagner DL, Sherman S, Lehman GA. Quality of life after bilateral thoracoscopic splanchnicectomy: long-term evaluation in patients with chronic pancreatitis. J Gastrointest Surg. 2002;6:845-852. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Buscher HC, Jansen JB, van Dongen R, Bleichrodt RP, van Goor H. Long-term results of bilateral thoracoscopic splanchnicectomy in patients with chronic pancreatitis. Br J Surg. 2002;89:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |