Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.4962
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: August 28, 2005
AIM: To study the protective effect of acupuncturing Tsusanli (ST36) on cold stress ulcer, and the expression of nitric oxide synthase (NOS) in hypothalamus and adrenal gland.
METHODS: Ulcer index in rats and RT-PCR were used to study the protective effect of acupuncture on cold stress ulcer, and the expression of NOS in hypothalamus and adrenal gland. Images were analyzed with semi-quantitative method.
RESULTS: The ulcer index significantly decreased in rats with stress ulcer. Plasma cortisol concentration was up regulated during cold stress, which could be depressed by pre-acupuncture. The expression of NOS1 in hypothalamus increased after acupuncture. The increased expression of NOS2 was related with stress ulcer, which could be decreased by acupuncture. The expression of NOS3 in hypothalamus was similar to NOS2, but the effect of acupuncture was limited. The expression of NOS2 and NOS3 in adrenal gland increased after cold stress, only the expression of NOS1 could be repressed with acupuncture. There was no NOS2 expression in adrenal gland in rats with stress ulcer.
CONCLUSION: The protective effect of acupuncturing Tsusanli (ST36) on the expression of NOS in hypothalamus and adrenal gland can be achieved.
- Citation: Sun JP, Pei HT, Jin XL, Yin L, Tian QH, Tian SJ. Effects of acupuncturing Tsusanli (ST36) on expression of nitric oxide synthase in hypothalamus and adrenal gland in rats with cold stress ulcer. World J Gastroenterol 2005; 11(32): 4962-4966
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/4962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.4962
Hypothalamus is a higher vegetative nerve center, which can accommodate not only the viscera activation, but also some important physiological functions, such as body temperature regulation, endocrine regulation and emotional response. Damage to this region would lead to acute gastric mucosal lesion.
Glands play an important role in the processes of stress. Psychological and physical stress may result in acute gastric mucosal lesions. It was reported that stress relates to nitric oxide (NO) catalyzed by nitric oxide synthase (NOS)[1,2]. Studies have demonstrated that NO also plays an important role in the healing process of both gastric and duodenal ulcers[3].
In this study, we focused on the protective effect of acupuncturing Tsusanli (ST36) on cold stress ulcer and the expression of NOS in hypothalamus and adrenal gland.
Twenty-two adult male Sprague-Dawley (SD) rats weighting 250±25 g were provided by Experimental Animal Center of PLA General Hospital.
The rats were divided into three groups randomly, 6 in control group, 8 in cold stress group (stress group), and 8 in pre-acupuncture group (pre-acupuncture group). Cold stress was induced in rats of stress group , pre-acupuncture group received pre-acupuncture treatment for 1 wk, before cold stress was induced. Control group received no treatment. Rats were housed at controlled temperature and humidity in 12 h light/dark cycle. The animals had free access to food and water.
SD rats were fasted for 24 h and water was deprived for 1 h before the experiments. The rats were fixed on board after being anesthetized with ether and then they were put into the cold room for 3 h.
The cold stress ulcer rat model was success fully established. There were point and line hemorrhage or superficial ulcer (1-2 mm in diameter).
The rats were placed into tailor-made mouse cages, and their bilateral legs were sufficiently exposed. Acupuncture at Tsusanli (ST36) was performed.
The rats were acupunctured with electrical needles (WQ1002K, electro-acupuncture equipment company, China) at 9-10 am, 30 min each time for 7 d. The frequency was 2-20 Hz. The original intensity was 2 V, and increased by 1 V every 10 min. No stimulation was given to the control group.
Rats were killed, hypothalamus and adrenal gland were preserved in liquid nitrogen for expression of NOS by reverse transcriptase-polymerase chain reaction (RT-PCR). Plasma cortisol levels were measured by specific radioimmunoassay (RIA). Biopsy mucosal samples were taken for the measurement of ulcer size, preserved and fixed in 40 mg/L formaldehyde solution for HE staining and then photographed under microscope.
The ulcer index calculation was expressed as[4] 1 score, if there was blotch erosion, 2 scores, when the erosion length was less then 1 mm, 3 scores, when the erosion length was 1-2 mm, 4 scores, when the erosion length was 2-3 mm, and 5 scores, when the erosion length was greater than 4 mm.
Total RNA was extracted using TRIzol one-step extraction method. Ultraviolet spectrophotometer was used to determine its concentration and purity.
All laboratory chemicals were obtained from Sigma Chemical Company.
cDNA was reverse transcripted using 20 μg total RNA, including Oligo dT (1.5 μL), dNTPs [25 mmol/(L·μL), 2 μL] and DEPC water (26 μL). In PCR procedure, 30 μL of the reaction system was used including 10× buffer (3 μL), dNTPs [2.5 mmol/(L·μL), 1.8 μL], sense primer [10 pmol/(L·μL), 1.2 μL], anti-sense primer [10 pmol/(L·μL), 1.2 μL], cDNA(1.2 μL/per tube) and MilliQ H2O (20.3 μL). Thirty-two cycles of RT-PCR were amplified at 94 °C for 30 s, at 58 °C for 30 s, and at 72 °C for 1 min.
The following primer sequences were used (Beijing SBS Company, China): rNOS1-sense: 5’> CGC AGA ACA CAT CAC AGG <3’; rNOS1-antisense:5’>AGA ACG GGG AGA AAT TCG <3’ (450-bp product);
rNOS2-sense: 5’>TGT TCC ATG CAG ACA ACC <3’; rNOS2-antisense: 5’>TTC AGA AGC AGA ATG TGA CC <3’( 407-bp product);
rNOS3-sense: 5’>CCT TTG ATC TCA ATG TCG< 3’; rNOS3-antisense: 5’>TAC GAA GAA TGG AAG TGG <3’ (374-bp product);
β-actin was used as control, its primer sequences: 5’>AAC CCT AAG GCC AAC CTG GAA AAG<3’ , and 5’>TCG TGA GGT AGT CTG TCA GGT<3’ (241-bp product).
After completion of the PCR amplifications, 10 μL of each PCR reaction was added to each lane of 2% agarose gel. The gel was then stained with 1 μg/mL ethidium bromide, viewed with a transilluminator, and photographed using UVP-GDS7600 gel image system to perform semi-quantitative analysis.
SPSS was used to perform statistical analysis and mapping. The data in experiments for ulcer index and plasma cortisol were presented as mean±SE, and analyzed by Student’s t-test for unpaired samples. Data for RT-PCR were analyzed by one-way analysis of variance (ANOVA) followed by a post hoc Duncan’s test. P<0.05 was considered statistically significant.
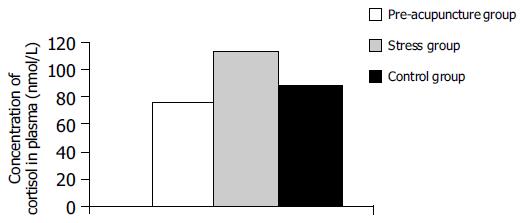
Plasma cortisol concentration was 104.38±8.31 in stress group, 66.83±12.25 in pre-acupuncture group, and 79.1±11.07 in control group. Difference was statistically significant in three groups (P<0.01 or P<0.05, Figure 1).
UI was 26.25±4.40 in stress group, 9.75±1.91 in pre-acupuncture group and 0 in control group (P<0.01) .
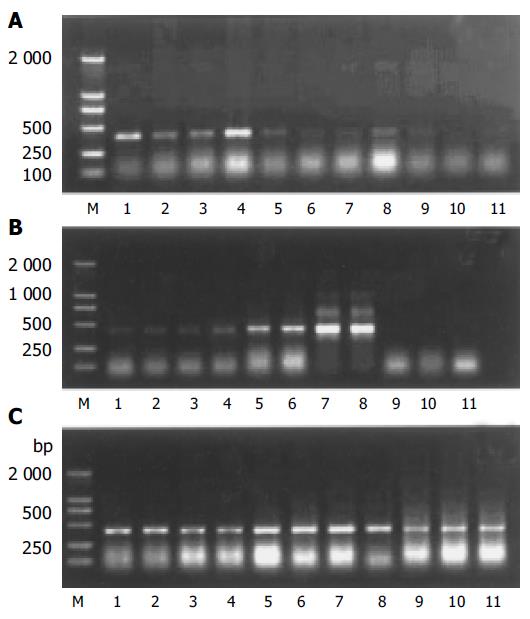
Expression of NOS1 mRNA The ratio of NOS1 mRNA to β-actin mRNA was 0.69±0.05 in control group, 0.77±0.09 in stress group, and 1.87±0.15 in pre-acupuncture group. There was a significant difference compared between acupuncture group and control group (P<0.01), but no significant difference was found between stress group and control group.
Expression of NOS2 mRNA The ratio of NOS2 mRNA to β-actin mRNA was 0.06±0.02 in control group, 0.24±0.05 in stress group, and 0.12±0.06 in pre-acupuncture group. There was a significant difference between pre-acupuncture group and stress group (P<0.01), as well as between stress group and control group(P<0.01).
Expression of NOS3 mRNA The ratio of NOS3 mRNA to β-actin mRNA was 0.30±0.08 in control group, 0.63±0.14 in stress group, and 0.45±0.14 in pre-acupuncture group. There was a significant difference between pre-acupuncture group and stress group (P<0.05), as well as between pre-acupuncture group and control group, stress group, and control group (P<0.05).
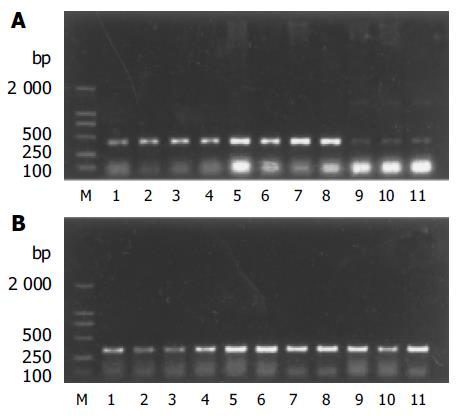
Expression of NOS1 mRNA The ratio of NOS1 mRNA to β-actin mRNA was 0.43±0.09 in control group, 0.98±0.23 in stress group, and 0.77±0.22 in pre-acupuncture group. There was a significant difference between pre-acupuncture group and stress group (P<0.05), as well as between stress group and control group (P<0.01), pre-acupuncture group and control group (P<0.01).
Expression of NOS2 mRNA There was no expression of NOS2 mRNA.
Expression of NOS3 mRNA The ratio of NOS3 mRNA to β-actin mRNA was 0.71±0.25 in control group, 2.54±1.15 in stress group, and 1.70×0.86 in pre-acupuncture group. There was a significant difference between stress group and control group (P<0.01), but no significance difference was found between pre-acupuncture group and stress group and between pre-acupuncture group and control group (P<0.05).
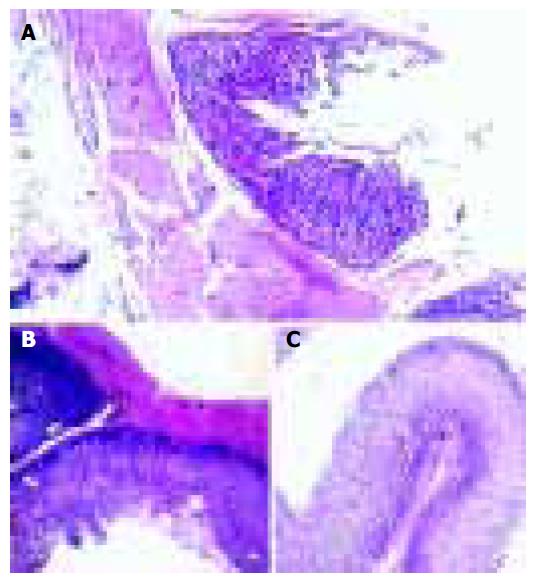
Interruption of continuity in gastric mucosa and submucosa, as well as damage in muscular layer were found in stress group. The continuity in gastric mucosa was slightly damaged, and its tissue morphology was relatively normal in pre-acupuncture group.
Studies have shown that the imbalance between NO and ET, decreases the gastric mucosal blood flow (GBF) and accelerates the gastric lesions[5,6]. NO has both protective and deleterious effects on gastric mucosa by different physiological and pathological mechanisms in various diseases. This double effect depends on the type of NOS involved in these pathological conditions[7].
It was reported that NO, as a neurotransmitter and a sensory neuropeptide, takes part in the physiological and pathological hyperemia reaction in gastric mucosa, increases gastric mucosal blood flow, thus protecting gastric mucosa[8,9]. Endogenetic NO plays an important role in maintaining vascular integrity and promoting the ulcer healing[10]. NO catalyzed by NOS, can function both as a second messenger and as a neurotransmitter, and also performs numerous physiological functions as an effective molecule. In pathological status, superfluous NO can increase vascular permeability, neuron decrease, neurotransmitter release and transitive obstacle, thus affecting organic physiological functions[11,12]. The damage of NO to gastric mucosa indicates, that higher concentrations of NO exhibit toxic effects through nitrosative and oxidative stress. The mechanism responsible for water immersion stress-induced gastric lesions in aged is related with the activity decrease of NOS in gastric mucosa[13].
Three types of NOS including neuronal NOS (NOS1), inducible NOS (NOS2) and endothelial NOS (NOS3) have been identified[14,15].
NOS1 is primarily expressed in neural cells and relates to many physiological functions of the central nervous system, such as synapse plasticity, neural regeneration, nerve conduction, neural development, and neuroendocrine regulation[16,17]. NOS2, also known as inducible NOS is typically expressed only after induction by pro-inflammatory cytokines and /or lipopolysaccharide (LPS). In a wide variety of pathological conditions, there is a notable biosynthesis of large quantities of NO, due to the increased expression of NOS2[18-20]. Both NOS2 and COX2 result in stress of rats[21]. NOS3 is primarily expressed in endothelial cells.
Both NOS1 and NOS3, also named as constitutive NOS (cNOS), are expressed in physiological status. NO catalyzed by NOS, mainly functions as a second messenger and a neurotransmitter. cNOS are related with gastric mucosa protection and the process of ulcer healing, In contrast, NOS2 affects the ulceration[22-24].
Stress could cause mucosal damage and increase vascular permeability. NOS inhibitor can aggravate these kinds of changes. L-arginine or endotoxin lipopolysaccharide can restrain the increase of vascular permeability[25,26]. In contrast, some studies reported that L-NAME (a nonspecific inhibitor of NOS) can delay mucosal lesions[27] by depressing bile acid secretion and ulcer index in stomach[28].
Results from the present studies, show that cold stress can differently affect NOS in hypothalamus. Acupuncture regulation for NOS is also different.
There was no significant difference in NOS1 in hypothalamus between stress group and control group, suggesting that NOS1 does not take part in stress reaction.
NOS1 expression in pre-acupuncture group was higher than that in control and stress groups, suggesting that puncturing Tsusanli (ST36) can upregulate the expression of NOS1, leading to synthesizing more NO, and protecting gastric mucosa against the damage induced by cold stress.
Different from NOS1, cold stress caused high expression of NOS3 compared to control group. Moreover, acupuncturing Tsusanli (ST36) could inhibit high expression of NOS3, suggesting that the mechanism of acupuncture in inhibiting NOS3 is different from NOS1. Acupuncturing Tsusanli (ST36) can, in part, downregulate the expression of NOS3, instead of those previously completely inhibiting its injurious expression. The above results are different from reported. Whether the peripheral reaction of NOS3 is different from that in central reaction needs to be confirmed.
The results of this study also showed that NOS in adrenal gland did not play a role as in hypothalamus. NOS3 takes part in the pathological process of cold stress ulcer, both in adrenal gland and in hypothalamus. Acupuncture can decrease the expression of NOS3. Although it can only be decreased in part, there is no statistical difference. The difference is that NOS1 takes part in the pathological stress process in adrenal gland. Acupuncture can down-regulate the pathological reaction.
The present experimental results display that NOS2 is highly expressed in hypothalamus, which is induced by cold stress. High expression of NOS2 leads to pathological changes in gastric mucosa[29,30]. Acupuncturing Tsusanli (ST36) can depress the overexpression of NOS2, thus leading to the protection of the body against injury. Studies have demonstrated that the expression of NOS2 is markedly upregulated after ulceration.
In the present study, we have confirmed that NOS2 participates in the pathological stress reaction, whereas NOS1 does not. NOS3 also participates in the pathological stress reaction, which is different from the previous reports[31,32]. It was reported that NOS1, NOS2, and NOS3 affect the healing of gastric ulcer[3].
In conclusion, NOS2 and NOS3 in hypothalamus participate in the pathological process of cold stress ulcer. Acupuncturing Tsusanli (ST36) can modulate the expression of NOS1 upward and depress the expression of NOS2 and NOS3, thus playing a role in protecting gastric mucous.
The authors thank Drs. Yi-Ming Mu and Ming Li for their help and advice.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Masood A, Banerji B, Vijayan VK, Ray A. Pharmacological and biochemical studies on the possible role of nitric oxide in stress adaptation in rats. Eur J Pharmacol. 2004;493:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | McLeod TM, López-Figueroa AL, López-Figueroa MO. Nitric oxide, stress, and depression. Psychopharmacol Bull. 2001;35:24-41. [PubMed] |
| 3. | Dixit C, Rastogi L, Dikshit M. Effect of nitric oxide modulators on pylorus-ligation-induced ulcers in the rat. Pharmacol Res. 1999;39:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Guth PH, Aures D, Paulsen G. Topical aspirin plus HCl gastric lesions in the rat. Cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology. 1979;76:88-93. [PubMed] |
| 5. | Zhang GF, Chen YR, Zhang MA. Nitrogen monoxide, endothelins and injury in gastric mucous membrane. Weichang Bingxue He Ganbingxue Zazhi. 1999;8; 293-296. |
| 6. | Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Drozdowicz D, Stachura J, Pajdo R, Hahn EG. Role of prostaglandins generated by cyclooxygenase-1 and cyclooxygenase-2 in healing of ischemia-reperfusion-induced gastric lesions. Eur J Pharmacol. 1999;385:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Cho CH. Current roles of nitric oxide in gastrointestinal disorders. J Physiol Paris. 2001;95:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Salomone S, Caruso A, Cutuli VM, Mangano NG, Prato A, Amico-Roxas M, Bianchi A, Clementi G. Effects of adrenomedullin on the contraction of gastric arteries during reserpine-induced gastric ulcer. Peptides. 2003;24:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Mohanakumar KP, Thomas B, Sharma SM, Muralikrishnan D, Chowdhury R, Chiueh CC. Nitric oxide: an antioxidant and neuroprotector. Ann N Y Acad Sci. 2002;962:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Ma L, Chow JY, Liu ES, Cho CH. Cigarette smoke and its extract delays ulcer healing and reduces nitric oxide synthase activity and angiogenesis in rat stomach. Clin Exp Pharmacol Physiol. 1999;26:828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Mishra OP, Delivoria-Papadopoulos M. Nitric oxide-mediated Ca++-influx in neuronal nuclei and cortical synaptosomes of normoxic and hypoxic newborn piglets. Neurosci Lett. 2002;318:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, Longo VD. Peroxynitrite mediates neurotoxicity of amyloid beta-peptide1-42- and lipopolysaccharide-activated microglia. J Neurosci. 2002;22:3484-3492. [PubMed] |
| 13. | Goto H, Tachi K, Hisanaga Y, Kamiya K, Ohmiya N, Niwa Y, Hayakawa T. Exacerbatory mechanism responsible for water immersion stress-induced gastric lesions in aged rats compared with young rats. Clin Exp Pharmacol Physiol. 2001;28:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Bolaños JP, Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim Biophys Acta. 1999;1411:415-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Barrachina MD, Panés J, Esplugues JV. Role of nitric oxide in gastrointestinal inflammatory and ulcerative diseases: perspective for drugs development. Curr Pharm Des. 2001;7:31-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Drew B, Leeuwenburgh C. Aging and the role of reactive nitrogen species. Ann N Y Acad Sci. 2002;959:66-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Sánchez S, Martín MJ, Ortiz P, Motilva V, Herrerías JM, Alarcón de la Lastra C. Role of prostaglandins and nitric oxide in gastric damage induced by metamizol in rats. Inflamm Res. 2002;51:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Higuchi Y, Hattori H, Hattori R, Furusho K. Increased neurons containing neuronal nitric oxide synthase in the brain of a hypoxic-ischemic neonatal rat model. Brain Dev. 1996;18:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Wada K, Chatzipanteli K, Busto R, Dietrich WD. Role of nitric oxide in traumatic brain injury in the rat. J Neurosurg. 1998;89:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Chavez AM, Morin MJ, Unno N, Fink MP, Hodin RA. Acquired interferon gamma responsiveness during Caco-2 cell differentiation: effects on iNOS gene expression. Gut. 1999;44:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Madrigal JL, García-Bueno B, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Relationship between cyclooxygenase-2 and nitric oxide synthase-2 in rat cortex after stress. Eur J Neurosci. 2003;18:1701-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | West SD, Helmer KS, Chang LK, Cui Y, Greeley GH, Mercer DW. Cholecystokinin secretagogue-induced gastroprotection: role of nitric oxide and blood flow. Am J Physiol Gastrointest Liver Physiol. 2003;284:G399-410. |
| 23. | Brzozowska I, Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Pajdo R, Drozdowicz D, Pawlik M, Ptak A, Hahn EG. Role of prostaglandins, nitric oxide, sensory nerves and gastrin in acceleration of ulcer healing by melatonin and its precursor, L-tryptophan. J Pineal Res. 2002;32:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Ohta Y, Nishida K. L-arginine protects against stress-induced gastric mucosal lesions by preserving gastric mucus. Clin Exp Pharmacol Physiol. 2002;29:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Hsieh JS, Wang JY, Lin SR, Lian ST, Chen FM, Hsieh MC, Huang TJ. Overexpression of inducible nitric oxide synthase in gastric mucosa of rats with portal hypertension: correlation with gastric mucosal damage. J Surg Res. 2003;115:24-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Araki H, Komoike Y, Matsumoto M, Tanaka A, Takeuchi K. Healing of duodenal ulcers is not impaired by indomethacin or rofecoxib, the selective COX-2 inhibitor, in rats. Digestion. 2002;66:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Fan YP, Chakder S, Gao F, Rattan S. Inducible and neuronal nitric oxide synthase involvement in lipopolysaccharide-induced sphincteric dysfunction. Am J Physiol Gastrointest Liver Physiol. 2001;280:G32-G42. [PubMed] |
| 28. | Chen M, Luo HS, Tong QY, Chen JH, Li XZ. Effects of pyloric local CGRP and NO on bile reflux in rat stomach with stress ulcer. Shijie Huaren Xiaohua Zazhi. 2004;12:2131-2134. |
| 29. | Chen K, Hirota S, Wasa M, Okada A. Expression of NOS II and its role in experimental small bowel ulceration in rats. Surgery. 1999;126:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Calatayud S, Ramírez MC, Sanz MJ, Moreno L, Hernández C, Bosch J, Piqué JM, Esplugues JV. Gastric mucosal resistance to acute injury in experimental portal hypertension. Br J Pharmacol. 2001;132:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Nishida K, Ohta Y, Ishiguro I. Preventive effect of teprenone on stress-induced gastric mucosal lesions and its relation to gastric mucosal constitutive nitric oxide synthase activity. Pharmacol Res. 1999;39:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Li Y, Wang WP, Wang HY, Cho CH. Intragastric administration of heparin enhances gastric ulcer healing through a nitric oxide-dependent mechanism in rats. Eur J Pharmacol. 2000;399:205-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |