Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.4957
Revised: December 1, 2004
Accepted: December 3, 2004
Published online: August 28, 2005
AIM: To evaluate the effects of dietary supplementation with vitamin E and selenium on proliferation and apoptosis of hepatic stellate cells (HSCs), in acute liver injury induced by CCl4, and to explore their role in the recovery from hepatic fibrosis phase.
METHODS: An acute liver damage model of rats was established by intraperitoneal injection of carbon tetrachloride (0.3 mL/100 g body weight) twice a week, then the rats were killed at 6, 24, 48, and 72 h after the first and third injection, respectively. A liver fibrosis model was established by the same injection for 8 wk. Then three rats were killed at 3, 7, 14, and 28 d after the last injection, respectively. The rats from the intervention group were fed with chow supplemented with vitamin E (250 mg/kg) and selenium (0.2 mg/kg), and the rats in the normal control group and pathological group were given standard chow. Livers were harvested and stained with hematoxylin and eosin, Sirius red. Activated HSCs were determined by α-smooth muscle actin immunohistochemistry staining. Apoptotic HSCs were determined by dual staining with the terminal deoxynucleotidyl transferase UTP nick end labeling (TUNEL) and α-smooth muscle actin immunohistochemistry. Serum alanine aminotransferase and aspartate aminotransferase were also analyzed.
RESULTS: In the acute liver damage model, the degree of liver injury was more serious in the pathological group than in the intervention group. At each time point, the number of activated HSCs was less in the intervention group than in the pathological group, while the number of apoptotic HSCs was more in the intervention group than in the pathological group. In the liver fibrosis model, the degree of liver fibrosis was more serious in the pathological group than in the intervention group. At each time point, the number of activated HSCs was less in the intervention group than in the pathological group, and the number of apoptotic HSCs was more in the intervention group than in the pathological group.
CONCLUSION: Vitamin E and selenium supplementation at the given level can inhibit CCl4-induced activation and proliferation of HSCs and promote the apoptosis of activated HSCs in acute damage phase. Vitamin E and selenium can also effectively decrease the degree of hepatic fibrosis and promote the recovery process.
- Citation: Shen XH, Cheng WF, Li XH, Sun JQ, Li F, Ma L, Xie LM. Effects of dietary supplementation with vitamin E and selenium on rat hepatic stellate cell apoptosis. World J Gastroenterol 2005; 11(32): 4957-4961
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/4957.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.4957
Liver fibrosis is characterized by increased deposition of extracellular matrix, and its mechanism is extremely complex. The common understanding about liver fibrosis is that the activation and proliferation of HSCs, a major source of extracellular matrix, play a key role in liver fibrogenesis[1,2]. Activated HSCs express α-SMA and are the major source of collagens and other matrix proteins which result in fibrosis. Resolution of liver fibrosis is associated with a net loss of activated HSCs mediated by apoptosis. Therefore, promoting HSC apoptosis may be a viable method to facilitate matrix degradation in fibrotic liver[3,4].
Studies have shown that oxidative stress is one of the mechanisms in the development of liver fibrosis[5-8], it is worth investigating the therapeutic role of antioxidative nutrients in hepatic fibrosis. A wealth of evidence indicates that antioxidative nutrients such as vitamin E and selenium can prevent liver fibrosis induced by carbon tetrachloride (CCl4)[9,10]. The major mechanism by which CCl4 induces rat liver fibrosis is that CCl3- results in a series of lipid peroxidation reactions. The activation of HSCs is enhanced with the increasing CCl4 concentration[11]. The results from our previous study confirmed, that dietary supplementation with vitamin E and selenium can protect hepatocytes and prevent liver fibrosis induced by CCl4 in rat model[12]. However, the mechanism of the effects is still not clear. Therefore, rat acute liver injury and hepatic fibrosis models were established by intraperitoneal injection of CCl4. Dietary supplementation with proper doses of vitamin E and selenium was given to rats in order to determine whether the antioxidative nutrients had an effect on the proliferation and apoptosis of activated HSCs during liver injury or recovery from liver fibrosis in vivo.
One hundred and fifty-six male Sprague Dawley rats (Shanghai Xipuer-bikai Company) weighing 250-300 g were randomly divided into normal control group, pathological group, and intervention group, 52 rats each group. The rats were fed with chow supplemented with vitamin E (250 mg/kg) and selenium (0.2 mg/kg diet) in the intervention group, and the rats in the other two groups were given standard chow. After 2 wk, the rats were injected with saline in the normal control group, and those in the other two groups were intraperitoneally injected with 0.3 mL/100 g body weight sterile CCl4 at 1:1 with olive oil twice a week. In the acute liver damage model, five rats were killed at 6, 24, 48, and 72 h after the first and third injection. In the liver fibrosis model, the same dose of CCl4 was given to rats for 6 wk, then the dose of CCl4 was reduced to 0.15 mL/100 g body weight for another 2 wk. Starting from the 8th wk, three rats were killed at 3, 7, 14, and 28 d after the last injection. Livers and serum were collected, one lobe from each liver was fixed in buffered formalin for histological observation. Serum alanine aminotransferase and aspartate aminotransferase were analyzed.
Liver sections were stained with hematoxylin and eosin, Sirius red. Pathological changes of the liver were observed . Five low power fields (×4) of each slide were randomly selected. The IMS color image integrate system was used to get the total area with Sirius red positive changes of these 5 views. The mean of area was calculated at each time point.
Serum was collected at each time point in the model of acute liver damage. Alanine aminotransferase and aspartate aminotransferase were tested by Beckman automatic clinic biochemistry system.
Activation of HSCs was associated with expression of α-smooth muscle actin (α-SMA). Immunostaining for α-SMA was used to detect and quantify activated HSCs in each specimen of the liver. The liver section of each rat at each time point was immunostained with horseradish peroxidase-conjugated secondary antibody (monoclonal anti α-SMA, Envision TM, DAKO Company) for its expression of α-SMA, and identified by DAB(brown). The number of α-SMA positive cells of each liver slide was counted in 30 randomly selected high power fields (×40) by two observers, one of whom was blinded. If the difference in results between the two observers was higher than 10%, the slides were recounted.
The liver section of each rat was treated with 0.3% H2O2, 180 μg/mL proteinase K, then fragmented DNA in apoptotic cells was detected by TUNEL technique. The Boehringer TUNEL staining kit(German) was used following the manufacturer’s instructions. TUNEL positive nuclei were identified by NBT/BCIP (violet) and immunostaining for α-SMA with horseradish peroxidase-conjugated secondary antibody (brown). Cells expressed both in TUNEL (with violet nuclei) and in immunostaining for α-SMA (with brown cell plasma) were considered as apoptotic HSCs. Each slide was then analyzed by two researchers as described above.
The data were expressed as mean±SD and analyzed by ANOVA. P<0.05 was considered statistically significant. All analyses were conducted by SPSS 10.0 statistical package. All the charts were drawn by excel 2000.
After injection of CCl4, livers of the rats were damaged and swollen and became yellow, perivenular ballooning degeneration was present, the pathological changes increased with the increase of time. At each time point, the lesion was more serious in the pathological group than in the intervention group.
Because there was no significant difference at different time points after the first or third injection of CCl4, the data were regrouped into one group for statistical analysis. The lesion in the pathological group was more serious than that in the intervention group, and became worse with the increase of time (Table 1).
| ALT (IU/L) | AST (IU/L) | |||
| After the first injection | After the third injection | After the first injection | After the third injection | |
| Intervention group (n = 20) | 289.6±80.7ab | 446.4±136.5b | 63±16.9ab | 219.9±98.2a |
| Pathological group (n = 20) | 396.5±134.4b | 781.6±295.7 | 85.3±57.9b | 362.5±129.6 |
| Normal control group (n = 20) | 256.4±71.6 | 194.1±69.7 | 53.9±20.1 | 58.5±25.0 |
Three days after the last injection of CCl4, the livers of rats were swollen, yellow, hard and had hepatocyte fatty degeneration with many fibrotic septae. The changes were more serious in the pathological group than in the intervention group. After 28 d of the last injection, the degree and areas of hepatocyte fatty degeneration decreased, compared to those in the normal control group.
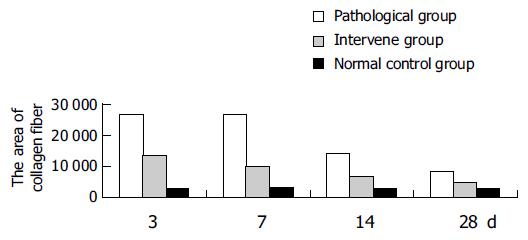
Also, because there was no significant difference at different time points after the first or third injection of CCl4, the data were regrouped into one group for statistical analysis. Collagen fibers in the intervention group were significantly more than those in the pathological group after the first and third injection of CCl4, but no significant difference was observed between the first and the third injection (Table 2).
In the liver fibrosis recovery model, the amount of collagen fibers 3, and 7 d after the last injection of CCl4 was much more than that in the model of acute liver injury. The amount of collagen fibers decreased with the changes of time, but was still more than that in the normal control group on d 28. At each time point, the amount of collagen fibers in the pathological group was more than that in the intervention group (Table 2, Figure 1).
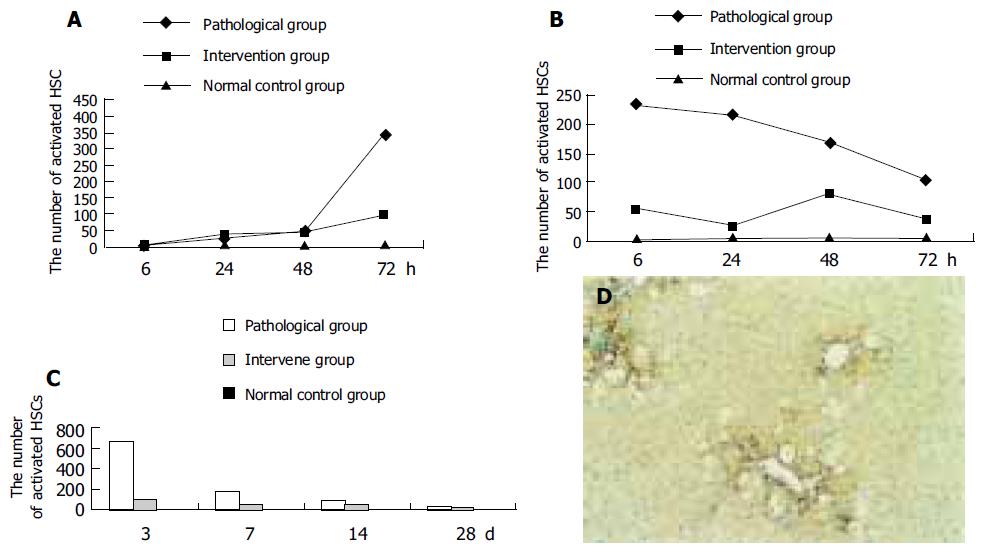
Twenty-four hours after the first injection of CCl4, the number of activated HSCs in the pathological and intervention groups began to increase, and reached the peak after 72 h. The number of activated HSCs began to decrease, 6 h after the third injection. The number of activated HSCs in the intervention group was less than that in the pathological group and more than that in the normal control group (Figures 2A, B).
In the pathological and intervention groups, the activated HSCs were clustered around the fibrotic septae. The number of activated HSCs decreased gradually 7 d after injection of CCl4, and was still more than that in the normal control group 28 d after injection of CCl4. At each time point, the number of activated HSCs in the intervention group was less than that in the pathological group (Figures 2C and D).
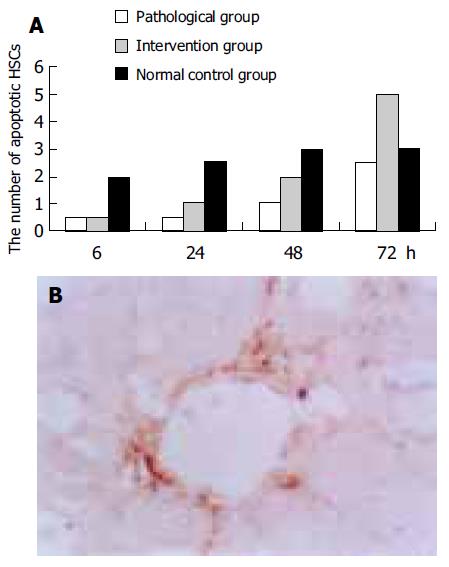
Twenty-four hours after the first injection of CCl4, the apoptotic HSCs began to increase gradually, and the number of apoptotic HSCs in the intervention group was more than that in the pathological group. As expected, 6, 24, 48 h after the first injection, the number of apoptotic HSCs in the intervention and pathological groups was less than that in the normal control group (Figure 3).
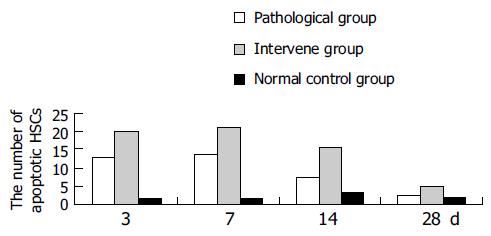
The apoptotic HSCs decreased gradually in the pathological and intervention groups after injection of CCl4, but was still more than that in the normal control group, 28 d after injection of CCl4. The number of apoptotic HSCs in the intervention group was more than that in the pathological group at each time point during liver fibrosis recovery, and was much more in the intervention and pathological groups than in the normal control group (Figure 4).
The dosage of CCl4, vitamin E, and selenium used in the study was selected as previously described[12-14]. Tests were carried out at different time points during acute damage and recovery, because it was reported that this is the best way to observe the continual changes of HSCs in vivo[15].
The results from histology and serum ALT and AST showed that vitamin E and selenium reduced liver damage during acute liver injury and had the effect of decreasing collagen fibers, thus preventing liver fibrosis. Hepatocyte damage or liver fibrosis as shown by many variables at each time point was different.
In the model of acute liver injury, although α-SMA immunostaining showed that the number of HSCs in the intervention group was less than that in the pathological group at each time point, Sirius red staining showed that the number of collagen fibers in the intervention group was more than that in the pathological and normal control groups, suggesting that vitamin E and selenium may promote the synthesis of collagen fibers. Since the synthesis of collagen also is a positive repairing response to hepatocyte injury, vitamin E and selenium can prevent liver damage during acute liver injury, not only by affecting HSCs but also by other ways. This needs to be confirmed by further studies.
The number of activated HSCs in the intervention group was less than that in the pathological group and more than that in the normal control group during acute damage phase. Meanwhile, the number of apoptotic activated HSCs in the intervention group was more than that in the pathological group and less than that in the normal control group, suggesting that CCl4 breaks the normal balance between activation and apoptosis of HSCs, resulting in more activation and lesser apoptosis. When the injury continues, more and more activated HSCs are generated thus producing more and more collagen fibers and leads to fibrosis. Vitamin E and selenium can inhibit the activation of HSCs and promote their apoptosis during acute damage phase, which helps to rebuild the balance between activation and apoptosis of HSCs, thus preventing liver fibrosis.
As expected, the number of apoptotic HSCs was more and that of activated HSCs was less than in the pathological group, suggesting that vitamin E and selenium can promote apoptosis of HSCs during recovery from liver fibrosis. During this stage, both activated and apoptotic HSCs in the pathological and intervention groups were more than those in the normal control group.
Iredale et al[15], have examined an experimental model of fibrosis during progressive and recovery phase to determine whether activated HSCs underwent apoptosis during spontaneous resolution of fibrosis in vivo, which is consistent with our results in the pathological group, suggesting that HSCs play a central role in the development and resolution of liver fibrosis.
Furthermore, the data in our study demonstrate that it needs more than 28 d for the rats to recover from hepatic fibrosis.
Activation and proliferation of HSC are the key to the formation of hepatic fibrosis, and apoptosis of HSCs is the crux of the recovery of fibrosis. Therefore, the disease can be intervened by inhibiting the activation of HSCs and inducing apoptosis. Our data show that vitamin E and selenium supplementation may be one of the mechanisms underlying the recovery of fibrosis[12]. Meanwhile, vitamin E and selenium may be an effective anti-fibrotic approach.
Co-correspondent: Liang-Min Xie
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 886] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 3. | Lee JI, Lee KS, Paik YH, Nyun Park Y, Han KH, Chon CY, Moon YM. Apoptosis of hepatic stellate cells in carbon tetrachloride induced acute liver injury of the rat: analysis of isolated hepatic stellate cells. J Hepatol. 2003;39:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Svegliati-Baroni G, Saccomanno S, van Goor H, Jansen P, Benedetti A, Moshage H. Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver. 2001;21:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Zhan Y, Wang Y, Wei L, Chen H. Effects of vitamin E on the proliferation and collagen synthesis of rat hepatic stellate cells treated with IL-2 or TNF-alpha. Chin Med J (Engl). 2003;116:472-474. [PubMed] |
| 7. | Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Lee KS, Lee SJ, Park HJ, Chung JP, Han KH, Chon CY, Lee SI, Moon YM. Oxidative stress effect on the activation of hepatic stellate cells. Yonsei Med J. 2001;42:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, Dianzani MU. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Bjørneboe A, Nenseter MS, Hagen BF, Bjørneboe GE, Prydz K, Drevon CA. Effect of dietary deficiency and supplementation with all-rac-alpha-tocopherol on hepatic content in rats. J Nutr. 1991;121:1208-1213. [PubMed] |
| 11. | Kim KY, Choi I, Kim SS. Progression of hepatic stellate cell activation is associated with the level of oxidative stress rather than cytokines during CCl4-induced fibrogenesis. Mol Cells. 2000;10:289-300. [PubMed] |
| 12. | Li F, Li XH, Cheng WF, Xie LM. Effects of antifibrosis and antioxidatation of dietary supplement vitamin E and selenium on experimental model of rats. Yingyang Xuebao. 2003;25:60-65. |
| 13. | Tsukamoto H, Matsuoka M, French SW. Experimental models of hepatic fibrosis: a review. Semin Liver Dis. 1990;10:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 193] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Zhang M, Song G, Minuk GY. Effects of hepatic stimulator substance, herbal medicine, selenium/vitamin E, and ciprofloxacin on cirrhosis in the rat. Gastroenterology. 1996;110:1150-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 828] [Article Influence: 30.7] [Reference Citation Analysis (0)] |