Published online Jan 21, 2005. doi: 10.3748/wjg.v11.i3.377
Revised: March 28, 2004
Accepted: April 13, 2004
Published online: January 21, 2005
AIM: Direct neoplastic invasion of esophageal inlet is an uncommon but significant sequela of advanced head and neck carcinomas. The aim of this study was to seek an optimal CT or MRI criterion for determining the neoplastic esophageal inlet involvement in order to help tumor staging and surgical planning.
METHODS: CT and MRI of 78 head and neck tumor cases were investigated retrospectively. At the level of the esophageal inlet on axial CT and MRI scans, the distance between the posterior aspect of cricoid cartilage and the anterior aspect of vertebra (d-CV) was measured by two senior radiologists who were unaware of clinical findings. Then, according to pathologic evidence and follow-up findings, these patients were divided into patient group, including 32 cases with neoplastic invasion of esophageal inlet and control group, including 46 cases without neoplastic esophageal inlet involvement. The statistical difference based on d-CV between the two groups was determined. The optimal criterion of d-CV on CT or MRI was assessed and its accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were evaluated respectively.
RESULTS: In control group, d-CV at the esophageal inlet level was 0.94±0.15 cm on axial CT and 0.91±0.18 cm on axial MRI, whereas in patient group, d-CV was 1.24±0.32 cm on CT and 1.31±0.36 cm on MRI. There was a statistical significance in d-CV between the two groups on CT and MRI modalities (P<0.01). d-CV greater than 1.0 cm was the typical feature of neoplastic invasion of the esophageal inlet with 73% sensitivity, 83% specificity, 79% accuracy, 76% PPV, 80% NPV on CT and 84% sensitivity, 77% specificity, 80% accuracy, 70% PPV, 88% NPV on MRI respectively.
CONCLUSION: Except for other CT and MR imaging features of neoplastic invasion of esophageal inlet, d-CV greater than 1.0 cm is an optimal adjunct criterion for esophageal inlet invasion by advanced head and neck carcinomas.
- Citation: Chen B, Yin SK, Zhuang QX, Cheng YS. CT and MR imaging for detecting neoplastic invasion of esophageal inlet. World J Gastroenterol 2005; 11(3): 377-381
- URL: https://www.wjgnet.com/1007-9327/full/v11/i3/377.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i3.377
Esophageal inlet is the pharyngoesophageal junction, which is the portion between postcricoid region of the hypopharynx and cervical esophagus. It is located at the level of the lower border of the cricoid cartilage or approximately the level of the sixth cervical vertebra[1,2]. Direct neoplastic invasion of esophageal inlet by advanced head and neck carcinomas is uncommon, but it is generally associated with upgrading primary tumor staging, increasing surgical complexity and a relatively low survival rate[2,27]. CT and MRI assessments of the presence or absence of esophageal inlet invasion by tumors is clinically useful for pretherapeutic staging, surgical planning and prediction of prognosis. Although there have been some literatures describing CT and MRI findings in the normal esophagus, esophageal tumor and invasion by advanced head and neck neoplasms[2-4,6,17,27], there are few articles on the role of CT or MRI for predicting esophageal inlet invasion by advanced head and neck carcinomas[2-4]. Therefore, the aim of this study was to seek an optimal criterion for determining the neoplastic esophageal inlet involvement in order to help tumor staging and surgical planning for resection and reconstruction.
Seventy-eight head and neck tumor cases, aged 27-81 years (mean age, 57.9 years), including 57 men and 21 women, were obtained from our hospital between January 1990 and February 2004. CT and MRI of these patients were investigated retrospectively by two senior radiologists without the knowledge of clinical evidence of esophageal inlet invasion. At the level of esophageal inlet on axial CT and MRI scans, the distance between the posterior aspect of cricoid cartilage and the anterior aspect of vertebra (d-CV) was measured. Then, according to pathologic evidence and follow-up findings, these patients were divided into patient group, including 32 cases with neoplastic invasion of esophageal inlet and control group, including 46 cases without neoplastic esophageal inlet involvement.
In patient group, 30 (93.8%) of the 32 cases received preoperative CT, whereas 19 (59.4%) underwent MR imaging. The presence of esophageal invasion was determined by pathologic evidence from surgical resection or biopsy. These cases included laryngeal squamous carcinoma (n = 9), postcricoid squamous carcinoma (n = 7), pyriform sinus squamous carcinoma (n = 10) and thyroid papillary or follicular carcinoma (n = 6).
In control group, 40 (87.0%) of the 46 cases underwent preoperative CT, whereas 30 (65.2%) received MR imaging. These 46 patients included laryngeal papilloma (n = 4), laryngeal squamous cell carcinoma (n = 17), pyriform sinus squamous cell carcinoma (n = 9), postcricoid squamous carcinoma (n = 2), thyroid papillary or follicular carcinoma (n = 9) and thyroid adenoma (n = 5). All these cases were confirmed without esophageal inlet invasion by head and neck surgeons during preoperative or operative evaluation and follow-up examinations.
Computed tomography was performed with scanners including Toshiba 600S, Toshiba Xvision/GX (Toshiba Medical Systems, Tochigi, Japan) and SOMATOM Sensation 4 (Siemens, Erlangen, Germany). Axial slices of 3-mm thickness and 3-mm interspace were obtained from the base of the tongue to the thoracic inlet before and after intravenous administration of meglumine iothalamate (Telebrix, Guerbet, Aulnay-sous-Bois, France).
MR imaging was performed using a 1.0-T unit (Schimadzu SMT-100 type, Shimadza Medical Systems, Tokyo, Japan) and a 1.5-T unit (Signa Performance Plus, GE Medical Systems, Milwaukee, WI) with anteroposterior volume neck coils. The MR imaging protocol consisted of axial T1-weighted spin-echo and T2-weighted spin-echo images. T1-weighted images included 450/18 [TR/TE] at 1.0 T and 550/10 [TR/TE] at 1.5 T, whereas T2-weighted images included 2000/90 [TR/TE] at 1.0 T and 4 200/95 [TR/TE] at 1.5 T. After intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Magnevist, Schering, Berlin, Germany), axial, sagittal and coronal T1-weighted spin-echo images were obtained. The slice thickness was 5 mm with no interslice gap.
At the level of the esophageal inlet on axial CT and MRI scans, d-CV was measured retrospectively. The results of d-CV between the two groups were analyzed by using Student’s t group test with SPSS software (SPSS, Chicago, IL). For all tests, statistical significance was set at P<0.05 level. Then, the optimal criterion of d-CV on CT or MRI was assessed by determining its accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) respectively.
Ovoid or concave normal esophageal inlet was found in 82.5% (33/40) of cases on CT and in 76.7% (23/30) of cases on MR imaging. The fat planes surrounding the well-defined margins of esophageal inlet and between the esophageal inlet and both lobes of the thyroid gland were visible in most CT or MRI scans. At the esophageal inlet level, d-CV was 0.94±0.15 cm on CT and 0.91±0.18 cm on MR imaging (Table 1).
| CT | MRI | |||
| Control group | Patient group | Control group | Patient group | |
| n | 40 | 30 | 30 | 19 |
| mean±SD (cm) | 0.94±0.15 | 1.24±0.32 | 0.91±0.18 | 1.31±0.36 |
| t | 4.806 | 4.544 | ||
| P | <0.01 | <0.01 | ||
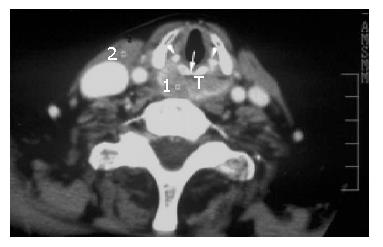
Postcricoid carcinoma with esophageal inlet invasion All the 7 cases of postcricoid tumor invading the esophageal inlet on CT and MRI scans were histologically documented squamous cell carcinoma, 85.7% (6/7) of the cases underwent preoperative CT scanning, whereas 57.1% (4/7) of the cases underwent MR imaging. On axial CT or MR images, all the cases had a large tumor mass arising from the postcricoid region resulting in asymmetric thickening of the pharyngeal wall and obliteration of surrounding fat plane (Figure 1), while 57.1% (4/7) of the cases presented with arytenoid cartilage destruction. On MR imaging, the sagittal, contrast-enhanced T1-weighted spin-echo (SE) image showed postcricoid tumor spreading circumferentially and towards the esophageal inlet and cervical esophagus. At the esophageal inlet level, d-CV was 1.25±0.31cm on CT and 1.28±0.45 cm on MR imaging (Table 2).
| Tumors | CT | MRI |
| Postcricoid carcinoma | 1.25±0.31 cm | 1.28±0.45 cm |
| (n=7) | (n=6) | (n=4) |
| Pyriform sinus carcinoma | 1.14±0.25 cm | 1.11±0.24 cm |
| (n=10) | (n=10) | (n=5) |
| Laryngeal carcinoma | 1.18±0.33 cm | 1.30±0.30 cm |
| (n=9) | (n=8) | (n=7) |
| Thyroid carcinoma | 1.46±0.38 cm | 1.72±0.39 cm |
| (n=6) | (n=6) | (n=3) |
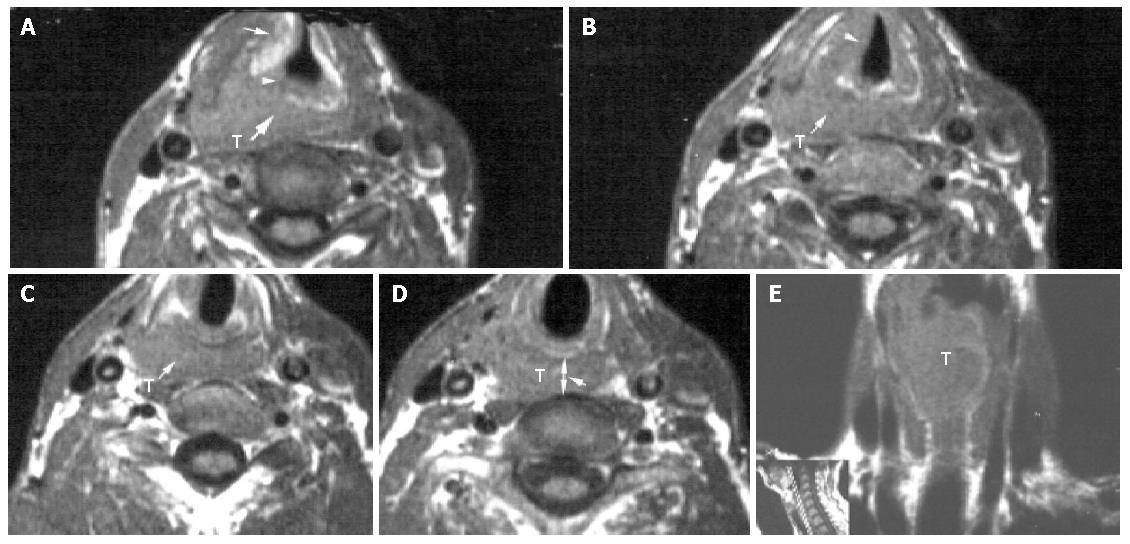
Pyriform sinus carcinoma with esophageal inlet invasion All the 10 cases of pyriform sinus tumor invading the esophageal inlet were histologically confirmed squamous cell carcinoma. Of the 10 cases, 5 (50%) involved the apex of pyriform sinus, 8 (80%) invaded submucously to the postcricoid region. 3 (30%) spread extensively to the aryepiglottic fold and epiglottis, 2 (20%) displayed thyroid cartilage erosion, 4 (40%) presented with arytenoid cartilage destruction. All the 10 cases showed a tumor mass as asymmetric thickening of the posterior wall. On MR imaging, all the 5 cases also revealed the tumor mass with poorly defined margins circumferentially around the esophageal inlet (Figure 2). At the esophageal inlet level, d-CV was 1.14±0.25 cm on CT ane 1.11±0.24 cm on MR imaging (Table 2).
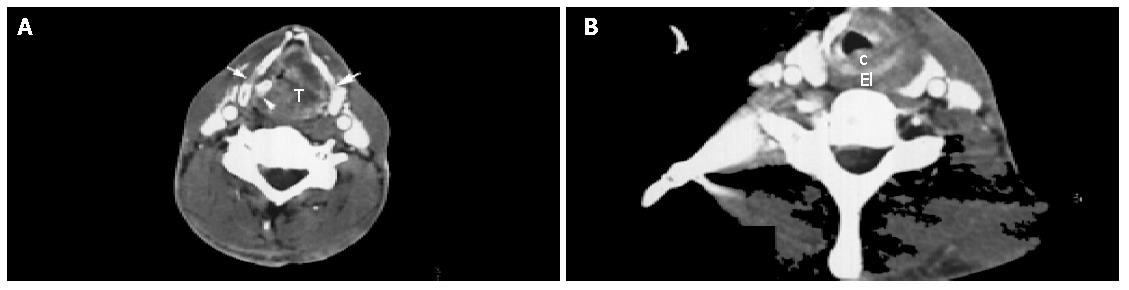
Laryngeal carcinoma with esophageal inlet invasion All the 9 cases of laryngeal carcinoma invading esophageal inlet were histologically documented squamous cell carcinoma. On MR imaging, 71.4% (5/7) of the cases showed a large tumor mass involving the whole larynx, obstructing the tracheal cavity and spreading posterolaterally to the esophageal inlet. The margin between tumor and esophageal inlet was poorly defined. On CT scanning, 25% (2/8) of cases indicated arytenoid cartilage erosion, whereas 37.5% (3/8) of cases revealed thyroid cartilage destruction (Figure 3). At the esophageal inlet level, d-CV was 1.18±0.33 cm on CT and 1.30±0.30 cm on MRI (Table 2).
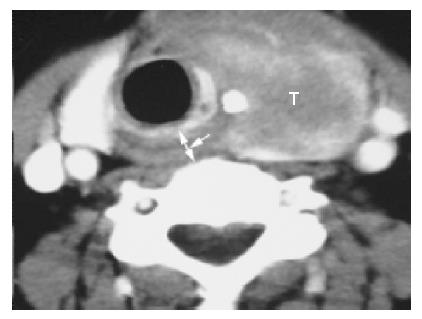
Thyroid carcinoma with esophageal inlet invasion All the 6 thyroid carcinoma cases ‘were preoperatively performed’ CT scanning, whereas 50% (3/6) of the cases underwent MR imaging. These 6 cases included 4 papillary carcinomas and 2 follicular carcinomas and were pathologically verified to have esophageal inlet involvement. Three (50%) of the 6 cases also indicated cervical esophageal invasion. On CT scans, the 6 cases showed a large tumor mass arising from the thyroid gland and invading the esophageal inlet (Figure 4). Of the 6 cases, 4 (66.7%) presented with contralateral displacement of the trachea, 2 (33.3%) revealed aortic invasion with concomitant luminal narrowing and effaceable flat plane between tumor and the aorta, 5 (83.3%) showed esophageal inlet involvement by a circumferential tumor mass greater than 120o, whereas 2 (33.3%) represented esophageal inlet invasion by a circumferential mass more than 180o. The margin between the esophagus inlet and thyroid mass was poorly defined. Two (33.3%) of the 6 cases presented with arytenoid cartilage erosion, whereas 1 (16.6%) of the 6 cases revealed thyroid cartilage destruction. On MR imaging, the extensive tumor mass displayed an intermediate, slightly lower or higher signal intensity on T1-weighted MR images and a high and heterogeneous signal intensity on T2-weighted MR images. At the esophageal inlet level, d-CV was 1.46±0.38 cm on CT and 1.72±0.39 cm on MR imaging (Table 2).
In the present work, we found that at the esophageal inlet level, d-CV was 0.94±0.15 cm on axial CT and 0.91±0.18 cm on axial MR imaging in control group, whereas d-CV was 1.24±0.32 cm on CT and 1.31±0.36 cm on MR imaging in patient group. There was a statistical significance in d-CV between these two groups (Table 1). d-CV greater than 1.0 cm was an optimal adjunct criterion of neoplastic invasion of the esophageal inlet with 73% sensitivity, 83% specificity, 79% accuracy, 76% PPV, 80% NPV on CT and 84% sensitivity, 77% specificity, 80% accuracy, 70% PPV, 88% NPV on MRI respectively (Table 3).
| Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive predictivevalue (%) | Negative predictivevalue (%) | |
| d-CV>1.0 cm on CT | 73 (22 of 30) | 83 (33 of 40) | 79 (55 of 70) | 76 (22 of 29) | 80 (33 of 41) |
| d-CV>1.0 cm on MRI | 84 (16 of 19) | 77 (23 of 30) | 80 (39 of 49) | 70 (16 of 23) | 88 (23 of 26) |
Esophageal inlet is the cricumferential ring extending from the postcricoid region of hypopharynx to the cervical esophagus. It is defined as the pharyngoesophageal junction which is delineated by the cricopharyngeus muscle[1]. Saleh et al[2] have further defined the esophageal inlet in their study to the point where the lower fibers of the inferior constrictor (cricopharyngeus) muscle are last seen attached to the posterolateral aspects of the laryngeal skeleton. They defined that the esophageal inlet was the portion of the postcricoid hypopharynx 3-mm or less above the lower margin of the cricoid cartilage. At this level, the emerging muscular wall of the esophagus merges with the lowermost fibers of the inferior constrictor (circophagngeus) muscle, whereas the flattened ellipsoid shape of the postcricoid hypopharynx gives way to a much more round ellipsoid shape of the esophageal inlet[1-4]. According to these previous literatures, we measured d-CV on axial CT and MRI sections at that level. In control group, d-CV of the normal esophageal inlet was 0.94±0.15 cm on axial CT and 0.91±0.18 cm on axial MRI.
Esophageal inlet invasion is a significant sequela of advanced head and neck carcinomas. In our study, malignancies associated with esophageal inlet included hypopharyngeal carcinoma, laryngeal carcinoma and thyroid carcinoma. Hypopharyngeal carcinomas, such as postcricoid carcinoma and pyriform sinus carcinoma, do have a propensity to infiltrate submucosally, either circumferentially or towards the esophageal inlet[2-5]. Laryngeal carcinoma could also spread extensively to hypopharynx and even to esophageal inlet[5]. Thyroid carcinoma could often invade the esophageal inlet by extrathyroidal growth rather than by nodal metastasis[6]. Nevertheless, esophageal involvement, including neoplastic invasion of esophageal inlet and cervical esophagus, is more often from extension of thyroid carcinoma than from spread of pharyngeal or laryngeal tumor[7]. Because of these characteristic spread patterns of advanced head and neck carcinomas to the esophageal inlet, the true extent only becomes apparent on axial CT and axial, coronal or sagittal MR images.
In recent years, the application of new diagnostic modalities such as endoscopic ultrasonography (EUS) and positron emission tomography (PET) on primary esophageal carcinoma has been widely investigated[8-16]. CT and MR imaging, however, still play a crucial role in the evaluation of primary esophageal tumors[4,17-20] and neoplastic invasion of esophagus by advanced head and neck carcinomas[2,7,21-23]. Roychowdhury et al[7] have reported that a circumferential mass or focal T2 signal abnormality on the esophageal wall on MR imaging suggests the presence of esophageal invasion by advanced head and neck carcinomas, whereas an intact fat plane, absence of wall thickening and no T2 wall signal abnormalities imply that the esophagus is not invaded. Wang et al[21] indicate that outer layer invasion and poorly defined margins are the significant factors of esophageal involvement due to thyroid carcinomas. They suggest that a MRI finding of outer layer invasion is optimal for diagnosing esophageal invasion by thyroid carcinoma. Nevertheless, there are a few studies on the role of CT or MRI in predicting esophageal inlet invasion by advanced head and neck carcinomas[2-4]. The accurate evaluation of patients at risk for esophageal inlet involvement requires specific criteria for determining tumor invasion at that level. Zhuang et al[24,25] have proposed that the distance between aryteoid cartilage and vertebra (d-AV) greater than 1.0 cm and the distance between crocoid cartilage and vertebra (d-CV) greater than 1.0 cm are the available criteria of neoplastic invasion of the retrocricoid region and esophageal inlet on CT and MRI modalities. However, the authors did not further describe these criteria in detail. In the present work, we found that except for other CT and MR imaging features of esophageal inlet involvement, such as circumscribed tumor mass adjacent to the esophageal inlet, poorly defined margins between the tumor and esophageal inlet and obliteration of the fat plane between the mass and esophageal inlet, d-CV at the esophageal inlet level might be another criterion, which was 1.24±0.32 cm on CT and 1.31±0.36 cm on MR imaging in patient group. There was statistical significance in d-CV between control group and patient group (Table 1). Therefore, we suggest that d-CV greater than 1.0 cm is a typical feature of neoplastic invasion of the esophageal inlet.
Predicting esophageal inlet invasion is important for determining primary tumor staging, predicting the prognosis and developing surgical strategy for advanced head and neck carcinomas. In patients with extensive head and neck tumors, esophageal inlet invasion would automatically upgrade primary tumor staging to a T4 classification[26]. Azurin et al[27] indicate that the overall 5-year survival for patients with stage IV squamous cell carcinoma is 24-27%. Kowalski et al[28] and McCaffrey et al[29] have reported that well-differentiated thyroid carcinoma usually has an excellent prognosis. However, when invasion of the upper aerodigestive tract occurs, it would be a source of significant morbidity as well as mortality for the patient. Thyroid carcinoma with minimal esophageal invasion could be treated by shave resection, whereas tumors with full-thickness and even intraluminal esophageal involvement could be treated by complete resection. Similar survival results after complete or shave resection and a poor survival after incomplete resection have been reported[28-30]. When the tumor just invades the full-thickness of cervical esophagus, pharyngo-esophagectomy is suggested with gastric transposition, and the larynx could be preserved. When the tumor invades the full-thickness of cervical esophagus, esophageal inlet and even hypopharynx, pharyngo-laryngo-esophagectomy are needed with gastric pull-up for reconstruction[28-30]. Furthermore, patients with advanced pharyngeal or laryngeal squamous cell carcinoma invading esophageal inlet might also benefit from surgical resection of the tumor for palliative maintenance of oral alimentation and prevention of airway compromise[27]. When the tumor just involves the esophageal inlet, limited esophagectomy is usually suggested and myocutaneous flap or jejunal interpositioning is usually required for closure. When the tumor extended to the cervical esophagus, esophagectomy is needed with gastric transposition or jejunal interposition used for reconstruction[2,27,31-35].
In conclusion, except for other CT and MR imaging features of neoplastic invasion of esophageal inlet, d-CV greater than 1.0 cm could be an optimal adjunct criterion to the esophageal inlet involvement by advanced head and neck carcinomas. It may exert a strong impact on the treatment recommendations for patients with esophageal inlet invasion by advanced head and neck carcinomas.
Edited by Kumar M and Wang XL
| 1. | Guo M. Applied anatomy of the hypopharyux and cervical esophagus. The modern theory and clinical practice of the Laryngeal and hypopharyngeal carcinoma. Jinan: Shandong Sci Tech Pub 2002; 31. |
| 2. | Saleh EM, Mancuso AA, Stringer SP. Relative roles of computed tomography and endoscopy for determining the inferior extent of pyriform sinus carcinoma: correlative histopathologic study. Head Neck. 1993;15:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Schmalfuss IM, Mancuso AA, Tart RP. Postcricoid region and cervical esophagus: normal appearance at CT and MR imaging. Radiology. 2000;214:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Schmalfuss IM. Imaging of the hypopharynx and cervical esophagus. Magn Reson Imaging Clin N Am. 2002;10:495-509, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Becker M. Larynx and hypopharynx. Radiol Clin North Am. 1998;36:891-920, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Machens A, Hinze R, Dralle H. Surgery on the cervicovisceral axis for invasive thyroid cancer. Langenbecks Arch Surg. 2001;386:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Roychowdhury S, Loevner LA, Yousem DM, Chalian A, Montone KT. MR imaging for predicting neoplastic invasion of the cervical esophagus. AJNR Am J Neuroradiol. 2000;21:1681-1687. [PubMed] |
| 8. | Räsänen JV, Sihvo EI, Knuuti MJ, Minn HR, Luostarinen ME, Laippala P, Viljanen T, Salo JA. Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol. 2003;10:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Kawano T, Nagai K, Nishikage T, Kumagai Y, Ogiya K, Tanaka K, Takeshita K. Progress of diagnosis for esophageal cancer. Gan To Kagaku Ryoho. 2003;30:909-913. [PubMed] |
| 10. | Kneist W, Schreckenberger M, Bartenstein P, Grünwald F, Oberholzer K, Junginger T. Positron emission tomography for staging esophageal cancer: does it lead to a different therapeutic approach? World J Surg. 2003;27:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kienle P, Buhl K, Kuntz C, Düx M, Hartmann C, Axel B, Herfarth C, Lehnert T. Prospective comparison of endoscopy, endosonography and computed tomography for staging of tumours of the oesophagus and gastric cardia. Digestion. 2002;66:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Ojima H, Tsukada K, Oriuchi N, Inoue T, Endo K. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer. 2002;94:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Rice TW. Clinical staging of esophageal carcinoma. CT, EUS, and PET. Chest Surg Clin N Am. 2000;10:471-485. [PubMed] |
| 14. | Meyenberger C, Fantin AC. Esophageal carcinoma: current staging strategies. Recent Results Cancer Res. 2000;155:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Wren SM, Stijns P, Srinivas S. Positron emission tomography in the initial staging of esophageal cancer. Arch Surg. 2002;137:1001-1006; discussion 1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Reed CE, Eloubeidi MA. New techniques for staging esophageal cancer. Surg Clin North Am. 2002;82:697-710, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Kumbasar B. Carcinoma of esophagus: radiologic diagnosis and staging. Eur J Radiol. 2002;42:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Okuda I, Kokubo T, Hoshihara Y, Udagawa H. Imaging diagnosis of esophageal carcinoma by computed tomography and magnetic resonance imaging. Nihon Geka Gakkai Zasshi. 2002;103:331-336. [PubMed] |
| 19. | Yamada I, Izumi Y, Kawano T, Yoshino N, Tetsumura A, Ohashi K, Shibuya H. Superficial esophageal carcinoma: an in vitro study of high-resolution MR imaging at 1.5T. J Magn Reson Imaging. 2001;13:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Hansen CP, Oskarsson K, Mortensen D. Computed tomography for staging of oesophageal cancer. Ann Chir Gynaecol. 2000;89:14-18. [PubMed] |
| 21. | Wang J, Takashima S, Matsushita T, Takayama F, Kobayashi T, Kadoya M. Esophageal invasion by thyroid carcinomas: prediction using magnetic resonance imaging. J Comput Assist Tomogr. 2003;27:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Takashima S, Matsushita T, Takayama F, Kadoya M, Fujimori M, Kobayashi T. Prognostic significance of magnetic resonance findings in advanced papillary thyroid cancer. Thyroid. 2001;11:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Zhuang QX, Gu YF, Wang W, Yang SX, Zhou JQ. Diagnosis of thyroid carcinoma with CT and MRI. Zhongguo Yixue Jisuanji Chengxiang Zazhi. 2000;6:386-388. |
| 24. | Zhuang QX, Gu YF, Wang W, Yang SX, Shang KZ, Yin SK. Imaging appearances of tumors invading esophageal inlet. Zhonghua Fangshexue Zazhi. 2001;35:116-119. |
| 25. | Zhuang QX. Imaging of tumors invading pharyngoesophageal junction. Zhongguo Yixue Jisuanji Chengxiang Zazhi. 2001;7:77-82. |
| 26. | Zhuang QX; UICC. TMN classification of malignant tumors. 5th ed. Springer-Verlag, Berlin, Heidelberg, New York. 1997;25-37. |
| 27. | Azurin DJ, Go LS, Kirkland ML. Palliative gastric transposition following pharyngolaryngoesophagectomy. Am Surg. 1997;63:410-413. [PubMed] |
| 28. | Kowalski LP, Filho JG. Results of the treatment of locally invasive thyroid carcinoma. Head Neck. 2002;24:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | McCaffrey JC. Evaluation and treatment of aerodigestive tract invasion by well-differentiated thyroid carcinoma. Cancer Control. 2000;7:246-252. [PubMed] |
| 30. | Ge JH, Zhao RL, Hu JL, Zhou WA. Surgical treatment of advanced thyroid carcinoma with aero-digestive invasion. Zhonghua Erbiyanhouke Zazhi. 2004;39:237-240. |
| 31. | Triboulet JP, Mariette C, Chevalier D, Amrouni H. Surgical management of carcinoma of the hypopharynx and cervical esophagus: analysis of 209 cases. Arch Surg. 2001;136:1164-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Bussi M, Ferrero V, Riontino E, Gasparri G, Camandona M, Cortesina G. Problems in reconstructive surgery in the treatment of carcinoma of the hypopharyngoesophageal junction. J Surg Oncol. 2000;74:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Bhathena HM. Free jejunal transfer for pharyngo-esophageal reconstruction. Acta Chir Plast. 2002;44:120-123. [PubMed] |
| 34. | Disa JJ, Cordeiro PG. Reconstruction of the hypopharynx and cervical esophagus. Clin Plast Surg. 2001;28:349-360. [PubMed] |
| 35. | Wadsworth JT, Futran N, Eubanks TR. Laparoscopic harvest of the jejunal free flap for reconstruction of hypopharyngeal and cervical esophageal defects. Arch Otolaryngol Head Neck Surg. 2002;128:1384-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |