Published online Jan 21, 2005. doi: 10.3748/wjg.v11.i3.353
Revised: December 30, 2003
Accepted: January 12, 2004
Published online: January 21, 2005
AIM: To study the effect of NS-398, a selective cyclooxygenase-2 (COX-2) inhibitor, on invasion of colon cancer cell line HT-29 in vitro and to explore its mechanisms.
METHODS: Invasive behaviors of the malignant colon cancer cell line HT-29 were investigated in this study. Expressions of COX-2 and CD44v6 in HT-29 cells were detected by flow cytometry. Cellular survival rate was determined by MTT assay. The invasive capacity was quantified by a modified Boyden chamber model. Alterations of cytoskeleton component F-actin were observed by confocal laser scanning microscope.
RESULTS: Flow cytometry analysis showed that COX-2 was highly expressed in HT-29 cells. The invasive capability of HT-29 cells could be greatly inhibited by NS-398 at the experimental concentrations of 0.1, 1.0 and 10 μmol/L with an inhibitory rate of 22.74%, 42.35% and 58.61% (P<0.01), respectively. MTT assay showed that NS-398 at the experimental concentrations had no significant influence on cellular viability, indicating that such anti-invasive effects had no relationship with cytotoxicity. F-actin was mainly distributed around nuclei forming annular structure in HT-29 cells. After exposure to NS-398 of 10 μmol/L, the annular structure around nuclei disappeared and the fluorescence intensity of F-actin decreased obviously. Treatment with NS-398 could down-regulate the expression of CD44v6 as well.
CONCLUSION: NS-398 has anti-invasive effects on colon cancer HT-29 cells in vitro, which may be mediated by a novel mechanism of disruption of cytoskeleton. Down-regulation of CD44v6 expression may be related to alterations of cytoskeleton.
- Citation: Jia XQ, Zhong N, Han LH, Wang JH, Yan M, Meng FL, Zhang SZ. Effect of NS-398 on colon cancer cells. World J Gastroenterol 2005; 11(3): 353-356
- URL: https://www.wjgnet.com/1007-9327/full/v11/i3/353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i3.353
Cyclooxygenase(COX), also known as prostaglandin endoper-oxidase or prostaglandin G/H synthase, is a rate-limiting enzyme involved in the conversion of arachidonic acid to prostaglandins. Two forms of cyclooxygenase, COX-1 and COX-2, have been identified. COX-1 is constitutively expressed in many tissues and responsible for various physiological functions, while COX-2 is an inducible enzyme, originally found to be induced by growth factors and other stimuli[1]. Recent studies have highlighted the relevance of COX-2 in human cancers, such as colorectal cancer, cholangiocarcinoma, liver cancer, esophageal carcinoma, and gastric cancer[2]. Recently clinical, cellular and animal experimental studies have indicated its relevance to tumor invasion and metastasis[3-6]. NS-398 is a selective COX-2 inhibitor and has a markedly reduced capacity of causing injury to gastrointestinal mucosa without inhibition of COX-1[7]. Some studies indicate that NS-398 could resist carcinogenesis through the effect of antiangiogenesis and proapoptosis[8,9]. Few studies have focused on anti-invasive and anti-metastatic effect of NS-398 on cancer, and its exact mechanisms still remain to be fully elucidated. We conducted the present study to investigate the effect of NS-398 on invasion of colon cancer cell line HT-29 in vitro and to elucidate its mechanisms.
Human colon cancer cell line HT-29 was obtained from Tumor Biological Treatment Center of Shandong Academy of Sciences and cultured in RPMI 1640 supplemented with 10% bovine calf serum (Gibco, USA) in a humidified atmosphere of 50 mL/L CO2 at 37 °C. Mouse NIH3T3 fibroblasts were supplied by Immunology Department of Shandong Academy of Sciences and maintained in DMEM supplemented with 10% calf bovine serum in an incubator. NS-398, MTT and BSA were obtained from Sigma Co. FITC-labelled mouse anti-human COX-2 MAb and FITC-labelled mouse anti-human IgG1 were provided by Cayman Chemical (USA). FITC-labelled mouse anti-human CD44v6 MAb was purchased from Pharmingen (USA). Matrigel was obtained from BD Company (USA). Alexa Flour 488 phalloidin was purchased from Molecular Probes (USA). Twenty-four-well Transwell chamber was purchased from Costar Co. Flow cytometry was a BD product (FACScan, USA). Confocal laser scanning microscope was a product from Bio-Rad (Radiance 2100, USA).
HT-29 cells at exponential phase were trypsinized, washed twice with PBS, suspended in PBS, stained with FITC-labelled mouse anti-human COX-2 MAb for 20 min in the dark and analyzed by flow cytometry. Cells treated with FITC-labelled mouse anti-human IgG1 were set as negative control. Fluorescence values were determined for each sample.
To quantify invasion, a modified Boyden chamber assay was used as described previously[10]. Polycarbonate filters with 8-μm pores of 24-well Transwell chamber were coated with matrigel (50 μg/pore) and placed at 37 °C for 1 h. HT-29 cells were treated with NS-398 at the concentrations of 0.1, 1.0 and 10 μmol/L, whereas control cells were just treated by the same amount of physiological saline. After 72 h of treatment, cells were harvested by trypsinization and added into the upper chamber (4×104 /chamber). Serum-free mouse NIH3T3 fibroblast conditioned medium was obtained by incubation of these cells for 24 h. This medium was used as a chemoattractant in the lower chamber. Following a 20-h incubation at 37 °C, cells in the upper chamber and the remaining cells on the upper surface of filters were mechanically removed. The filters were fixed with 40 mg/L formaldehyde, and the cells on the lower surface were counted under inverted microscope at 200×magnification. Five fields were counted per filter, and the experiment was done in triplicate. Invasive activity was assessed by the average number of cells migrated to the lower surface of the filter. We found NS-398 had a significant anti-invasive effect on HT-29 cells if the inhibitory rate (IR) was higher than 30%, which showed a significant difference (P<0.05) compared with control. IR was calculated according to the formula: IR = (number of invasive cells of control group-number of invasive cells of experimental group)/ number of invasive cells of control group×100%.
HT-29 cells were plated (2×103 per well) in 96-well plates and incubated for 24 h. Medium containing 0, 0.1, 1.0 and 10 μmol/L of NS-398 was put to the cells of different experimental groups in six parallel wells. Seventy-two hours later, 20 μL of 5 mg/mL MTT was added to each well. After incubation at 37 °C for 4 h, the MTT medium was removed and 100 μL of DMSO was added. Color reaction was measured by a spectrometer at the wavelength of 570 nm. Cell viability was assessed by the ratio of absorbance of NS-398-treated cells to that of controls.
HT-29 cells were put in a 24-well plate and allowed to attach overnight. After treatment with different concentrations of NS-398 for 72 h, the cells were washed twice with PBS (pH7.4) and then fixed with 37 mL/L formaldehyde for 10 min at room temperature. Then the cells were washed twice and made permeable with 0.1% Triton X-100 in PBS for 5 min. The cells were washed twice with PBS and 1% BSA was added for 20 min to reduce nonspecific background staining, washed twice again and then 200 μL of phalloidin in PBS (5 U/mL) was added for 30 min in the dark. A little PBS was left in the 24-well tissue culture dishes and the specimens were analyzed by confocal laser scanning microscope.
After treating with different concentrations of NS-398, HT-29 cells were harvested by mild trypsinization. Expression of CD44v6 in HT-29 cells was detected by flow cytometry as previously; the only difference was that FITC-labelled mouse anti-human CD44v6 mAb was used instead of FITC-labelled mouse anti-human COX-2 mAb.
All statistical analyses were carried out with SPSS 10.0 software. The statistical significance was evaluated by Student’s t test. P<0.05 was considered statistically significant.
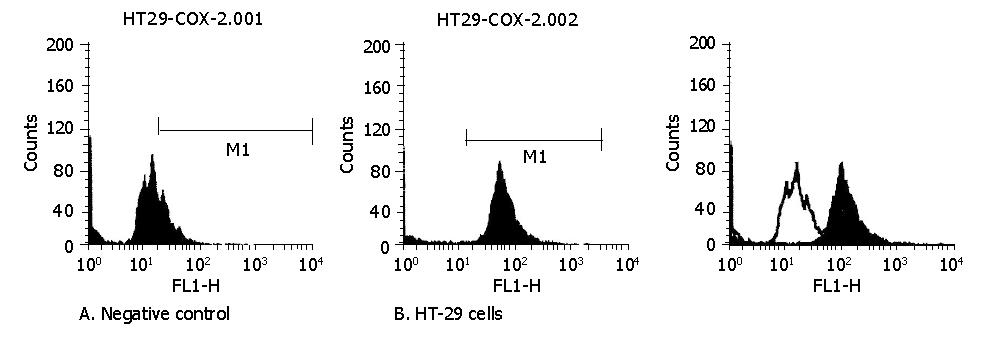
The fluorescent intensity of COX-2 was 10.92±1.55 in negative control while 66.07±7.66 in HT-29 cells. The difference between these two groups was significant (P<0.01) suggesting that COX-2 was positively expressed in HT-29 cells (Figure 1).
The cancer cell invasion was significantly suppressed 72 h after NS-398 treatment at the concentrations of 0.1, 1.0 and 10 μmol/L with an inhibitory rate of 22.74%, 42.35% and 58.61%, respectively. Statistical analysis showed that the difference between experimental group and untreated cells was significant (P<0.01). As IR in cells treated with 0.1 μmol/L was lower than 30%, we inferred 0.1 μmol/L NS-398 had no anti-invasive effect on HT-29 cells. In contrast, 1.0, 10 μmol/L NS-398 could significantly inhibit the invasive ability of HT-29 cells in vitro (Table 1).
The cell survival rate of HT-29 treated with 0.1, 1.0 and 10 μmol/L NS-398 for 72 h was 94.8%, 92.57% and 91.56% respectively without significant difference compared with control (P>0.05). This indicated that NS-398 had no significant effect on cell viability at experimental concentrations.
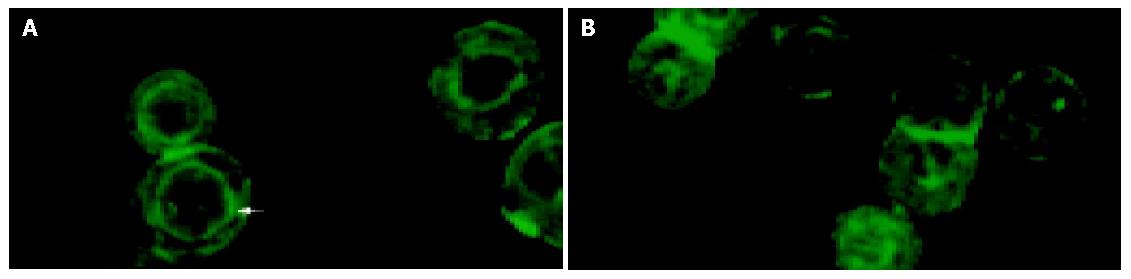
As shown in Figure 2, actin cytoskeleton was visualized by fluorescence microscopy and stained by phalloidin. F-actin was mainly distributed around nuclei to take on annular structure in the untreated control cells. Exposure of HT-29 cells to relatively lower concentrations of NS-398 (0.1 μmol/L and 1.0 μmol/L) had no significant effect on F-actin. However, at the highest dose of NS-398 (10 μmol/L), the annular structure around nuclei disappeared and the fluorescence intensity of F-actin was obviously decreased. Part of F-actin was depolymerized and dispersed in cytoplasm.
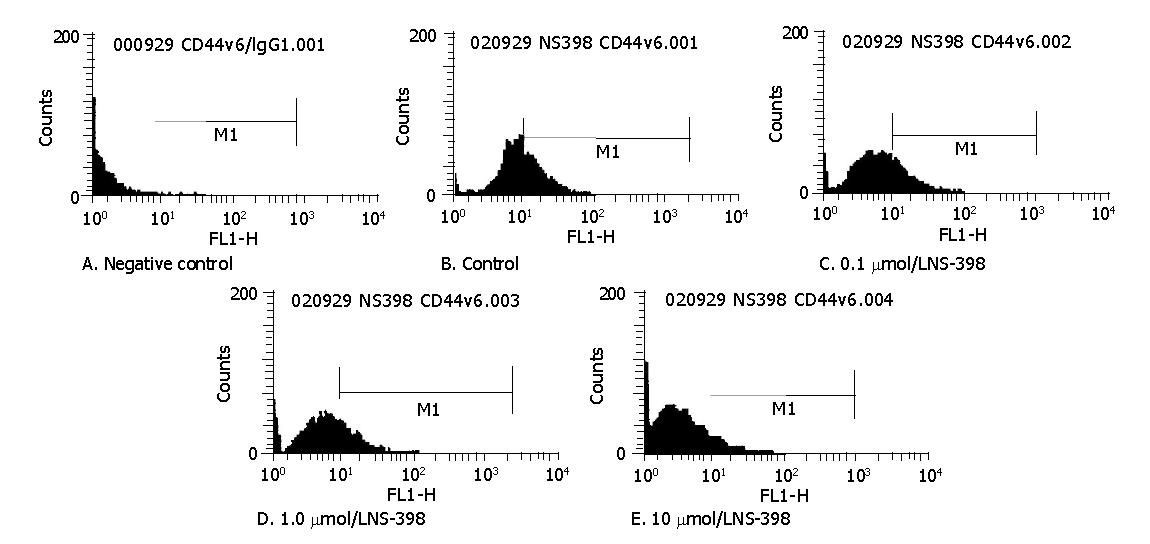
The fluorescence intensity of CD44v6 was 1.09±0.18 in negative control and 11.37±1.05 in HT-29 cells. The difference between these two groups was significant (P<0.01) suggesting that CD44v6 was positively expressed in HT-29 cells. After treatment with 0.1 μmol/L NS-398, the fluorescence intensity of CD44v6 was 9.71±1.45 without significance compared with untreated cells (P>0.05). After exposure to 1.0 and 10 μmol/L NS-398, the fluorescence intensity of CD44v6 was 8.0833±1.2070 and 4.3667±0.6834 respectively. The differences between the two groups and control group were significant (P<0.01). We found that the expression of CD44v6 in HT-29 cells significantly decreased in a dose-dependent manner (Figure 3).
Colon cancer is one of the common malignant tumors in China. Invasion and metastasis are two major causes leading to a fatal result. By invading the normal tissues, cancer cells could adhere to extracellular matrix (ECM) and migrate through degraded ECM into circulation[11]. This invasive growth is required for metastasis of cancer. Recent studies have specifically addressed the relationship between COX-2 level and invasion and metastasis of colorectal carcinoma. It has been found that high COX-2 expression is associated with invasion and metastasis of colon carcinoma[12,13]. In our study, we found that COX-2 was highly expressed in HT-29 cells. HT-29 cells could degrade and migrate through ECM component matrigel, which indicates their malignant behavior. These findings are in agreement with previous studies[12,13]. After treatment with NS-398 at 0.1, 1.0 and 10 μmol/L, the number of invasive cells significantly decreased compared with control group in a dose-dependent manner (P<0.01). The inhibitory rate was 22.74% (<30%), 42.35% and 58.61% respectively. We inferred NS-398 at 1.0 μmol/L and 10 μmol/L could significantly suppress the invasive ability of HT-29 cells in vitro. A number of researches[14,15] have found that selective COX-2 inhibitors could inhibit cell division and alter cell cycle distribution in cultured cancer cells. Tumor cells are arrested in G0/G1 phase with reduced cell cycle progression in G1-S transition, finally undergoing apoptosis. In order to determine whether such inhibitory effects of NS-398 were related to its cytotoxicity or not, cell viability was detected by MTT assay. We found NS-398 had no significant effect on cell viability at the experimental concentrations, indicating that anti-invasive effect of NS-398 on HT-29 cells does not result from its cytotoxicity. Despite these clues, the role of selective COX-2 inhibitor in suppressing the invasion and metastasis of colon cancer is still not understood.
Actin is a major component of cytoskeleton and exists in cytoplasm as F-actin and G-actin with homeostasis. It has been found that actin is involved in many cellular functions, including cellular motility, maintenance of cell shape, adhesion to ECM and cell invasion[16-21]. Alterations in organization and polymerization of actin have been known to accompany malignant transformation of many cell types. Thus, actin seems to be critical for migration and invasion of cancer cells. Selective targeting of actin filaments in cancer chemotherapy seems to be a very attractive approach. Up to now, we have not found reports about the effect of selective COX-2 inhibitor on cytoskeleton of cancer cells. In the current study cytoskeleton F-actin was investigated by confocal laser scanning microscope. We found that F-actin was mainly distributed around nuclei to take on annular structure in the untreated control cells. NS-398 at lower concentrations (0.1 and 1.0 μmol/L) had no significant effect on actin stress fibers. At the highest dose of 10 μmol/L, NS-398 could disrupt the architecture of F-actin and notably decrease fluorescence intensity of F-actin. Part of F-actin was depolymerized and dispersed in the cytoplasm. We think that disruption of actin cytoskeleton might be a novel mechanism of anti-invasive effect of NS-398 on HT-29 cells in vitro. Li et al blocked HT-29 cells with anti-CD44v6 monoclonal antibodies, and found the same changes of actin cytoskeleton and decreased invasion of HT-29 cells as ours. They concluded CD44v6 might affect the distribution, polymerization and depolymerization of actin of HT-29 cells, thus suppressing metastasis. Using flow cytometry, we found CD44v6 was positive in HT-29 cells and NS-398 could down-regulate the expression of CD44v6 in a dose-dependent manner. Therefore we believe that down-regulation of CD44v6 expression is related to disruption of cytoskeleton of HT-29 cells after treatment with NS-398.
In conclusion, NS-398 exerts its anti-invasive effect on HT-29 cells in vitro, possibly by disrupting the cytoskeleton. Disruption of cytoskeleton may be a novel mechanism involved in anti-invasive effect of NS-398 on colon cancer cells. In addition, NS-398 could down-regulate CD44v6 expression dose-dependently, which may be related to the disruption of cytoskeleton by NS-398. NS-398 is a promising agent for inhibiting invasion and metastasis of colon cancer.
Edited by Zhu LH and Wang XL Proofread by Chen WW
| 1. | Dohadwala M, Luo J, Zhu L, Lin Y, Dougherty GJ, Sharma S, Huang M, Pold M, Batra RK, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001;276:20809-20812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Kawabe A, Shimada Y, Uchida S, Maeda M, Sato F, Itami A, Imamura M. Expression of cyclooxygenase-2 is associated with carcinogenesis of the lower part of thoracic esophageal squamous cell carcinoma and p53 expression. Oncology. 2002;62:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Kim HJ, Wu HG, Park IA, Ha SW. High cyclooxygenase-2 expression is related with distant metastasis in cervical cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:16-20. |
| 4. | Buskens CJ, Van Rees BP, Sivula A, Reitsma JB, Haglund C, Bosma PJ, Offerhaus GJ, Van Lanschot JJ, Ristimäki A. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Tsujii M, Kuwano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. [RCA] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1039] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 6. | Yamauchi T, Watanabe M, Hasegawa H, Nishibori H, Ishii Y, Tatematsu H, Yamamoto K, Kubota T, Kitajima M. The potential for a selective cyclooxygenase-2 inhibitor in the prevention of liver metastasis in human colorectal cancer. Anticancer Res. 2003;23:245-249. [PubMed] |
| 7. | Barnett J, Chow J, Ives D, Chiou M, Mackenzie R, Osen E, Nguyen B, Tsing S, Bach C, Freire J. Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim Biophys Acta. 1994;1209:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 243] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, Chung SC, Sung JJ. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003;23:1317-1322. [PubMed] |
| 9. | Minter HA, Eveson JW, Huntley S, Elder DJ, Hague A. The cyclooxygenase 2-selective inhibitor NS398 inhibits proliferation of oral carcinoma cell lines by mechanisms dependent and independent of reduced prostaglandin E2 synthesis. Clin Cancer Res. 2003;9:1885-1897. [PubMed] |
| 10. | Attiga FA, Fernandez PM, Weeraratna AT, Manyak MJ, Patierno SR. Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res. 2000;60:4629-4637. [PubMed] |
| 11. | Mammoto T, Mukai M, Mammoto A, Yamanaka Y, Hayashi Y, Mashimo T, Kishi Y, Nakamura H. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin J, Yang WK. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer. 2001;91:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Kakiuchi Y, Tsuji S, Tsujii M, Murata H, Kawai N, Yasumaru M, Kimura A, Komori M, Irie T, Miyoshi E. Cyclooxygenase-2 activity altered the cell-surface carbohydrate antigens on colon cancer cells and enhanced liver metastasis. Cancer Res. 2002;62:1567-1572. [PubMed] |
| 14. | Hu KQ, Yu CH, Mineyama Y, McCracken JD, Hillebrand DJ, Hasan M. Inhibited proliferation of cyclooxygenase-2 expressing human hepatoma cells by NS-398, a selective COX-2 inhibitor. Int J Oncol. 2003;22:757-763. [PubMed] |
| 15. | Narayanan BA, Condon MS, Bosland MC, Narayanan NK, Reddy BS. Suppression of N-methyl-N-nitrosourea/testosterone-induced rat prostate cancer growth by celecoxib: effects on cyclooxygenase-2, cell cycle regulation, and apoptosis mechanism(s). Clin Cancer Res. 2003;9:3503-3513. [PubMed] |
| 16. | Donald CD, Cooper CR, Harris-Hooker S, Emmett N, Scanlon M, Cooke DB. Cytoskeletal organization and cell motility correlates with metastatic potential and state of differentiation in prostate cancer. Cell Mol Biol (Noisy-le-grand). 2001;47:1033-1038. [PubMed] |
| 17. | Akisaka T, Yoshida H, Inoue S, Shimizu K. Organization of cytoskeletal F-actin, G-actin, and gelsolin in the adhesion structures in cultured osteoclast. J Bone Miner Res. 2001;16:1248-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 441] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Verschueren H, Van der Taelen I, Dewit J, De Braekeleer J, De Baetselier P. Metastatic competence of BW5147 T-lymphoma cell lines is correlated with in vitro invasiveness, motility and F-actin content. J Leukoc Biol. 1994;55:552-556. [PubMed] |
| 20. | Iwazaki R, Watanabe S, Otaka K, Ota K, Ono Y, Sato N. The role of the cytoskeleton in migration and proliferation of a cultured human gastric cancer cell line using a new metastasis model. Cancer Lett. 1997;119:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Haier J, Nicolson GL. Role of the cytoskeleton in adhesion stabilization of human colorectal carcinoma cells to extracellular matrix components under dynamic conditions of laminar flow. Clin Exp Metastasis. 1999;17:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |