Published online Jan 21, 2005. doi: 10.3748/wjg.v11.i3.340
Revised: March 28, 2004
Accepted: April 13, 2004
Published online: January 21, 2005
AIM: It has been reported that regular consumption of nonsteroidal anti-inflammatory drugs like indomethacin decreases the incidence and mortality rate of a number of gastrointestinal cancers. We aimed to explore the efficacy and possible mechanisms of indomethacin on tumor growth and tumor angiogenesis of human colon cancer xenografts in nude mice.
METHODS: MTT (thiazolyl blue) assay was used to assess the effect of indomethacin on cultured human colorectal cancer cell line HCT116. HCT116 cells were inoculated subcutaneously into BALB/c-nu/nu mice. After oral administration of indomethacin, 3 mg/kg.d for 4 wk, animals were sacrificed by cervical dislocation. Immunohistochemical staining was employed to determine the microvessel density (MVD) and vascular endothelial growth factor (VEGF) expression in tumor tissues.
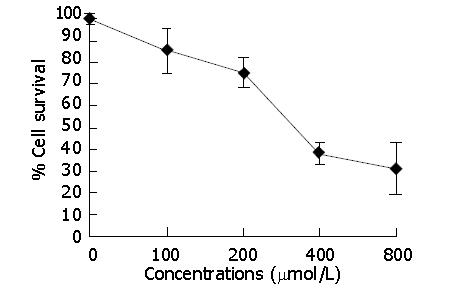
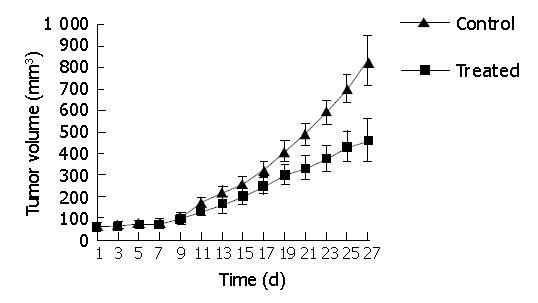
RESULTS: Indomethacin, a non-selective COX inhibitor, significantly decreased the viability of HCT116 cells in a dose-dependent manner (P<0.05) with 50% inhibition at approximately 318.2±12.7 μmol/L. Growth of HCT116 cell tumor was significantly suppressed by indomethacin. The tumor volume was significantly decreased in the treated group (458.89±32.07 mm3) compared to the control group (828.21±31.59 mm3) (P<0.05). The MVD of the treated group (19.50±5.32) was markedly decreased compared to the control group (37.40±4.93) (P<0.001). The VEGF expression of the treated group (1.19±0.17) was obviously reduced as compared to the control group (1.90±0.48) (P<0.01). The decrease in MVD was positively correlated with the decrease of VEGF expression (rs = 0.714, P<0.05). We did not see gastrointestinal complications in the treated group and no differences were noted in the body weight of the mice between the two groups throughout the study (P>0.05).
CONCLUSION: Indomethacin can significantly decrease the viability of cultured HCT116 cells and retard human colorectal HCT116 cell tumor growth via inhibiting tumor angiogenesis, which might be through reduction of VEGF expression.
- Citation: Wang HM, Zhang GY. Indomethacin suppresses growth of colon cancer via inhibition of angiogenesis in vivo. World J Gastroenterol 2005; 11(3): 340-343
- URL: https://www.wjgnet.com/1007-9327/full/v11/i3/340.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i3.340
Tumor angiogenesis or the formation of new vessels within tumors, plays a pivotal role in the development of carcinomas in various tissues[1]. Tumor cells need blood vessels to grow greater than 2-3 mm and to extend to adjacent tissues[2]. Therefore, suppression of tumor angiogenesis has recently become a central focus of cancer therapy. Indomethacin belongs to nonsteroidal anti-inflammatory drugs (NSAIDs). A broad spectrum of data has demonstrated that regular consumption of NSAIDs can reduce the morbidity and mortality of colorectal cancer[3-10], with one of its possible mechanisms being inhibition of angiogenesis[11-13]. In the present study, we examined the inhibitory effect of indomethacin on cultured human colon cancer cell line HCT116, and evaluated tumor growth and angiogenesis of HCT116 xenografts in BALB/c nude mice treated with indomethacin.
Indomethacin, thiazolyl blue (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Corporation (USA). Stock solution of indomethacin was made at 200 μmol/L in DMSO and stored at -20 °C. The final concentration of DMSO for all treatments including the negative control was maintained at 5 mL/L. The process was done in subdued light just prior to administration.
RPMI 1640 culture medium, fetal calf serum (FCS) and trypsin were obtained from Cell Center of Xiangya School of Medicine, Central South University (Changsha, China).
Monoclonal mouse anti-CD34 and anti-VEGF were products of Santa Cruz (USA). HistostainTM-streptavidin/peroxidase (SP) kit was purchased from Zhongshan Biotechnology Corporation (Beijing, China).
Human colon cancer cell line HCT116 (American Type Culture Collection, ATCC) was maintained in RPMI 1640 medium supplemented with 100 mL/L heat-inactivated FCS at 37 °C in a humidified atmosphere containing 50 mL/L CO2. Subconfluent HCT116 cells were dissociated with 2 g/L trypsin-0.2 g/L EDTA and suspended in RPMI 1640 serum-free medium at a density of 1.0×108 cells/mL. Only single cell suspensions of greater than 95% viability as determined by trypan blue exclusion were used for experiments.
Four-six-week old male BALB/c-nu/nu mice, weighing 16-18 g, were purchased from Experimental Animal Center of Shanghai. The mice were housed under specific pathogen-free conditions using laminar flow racks and given sterilized food and water. All animal experiments were approved by the Institutional Animal Care and Use Committee.
Viable HCT116 cells were seeded in 96-well microtiter plates at a concentration of 2×104 cells/well. After a 24-h preincubation at 37°C in a humidified atmosphere containing 50 mL/L CO2, the medium was aspirated. Various concentrations of indomethacin (100 μmol/L, 200 μmol/L, 400 μmol/L, and 800 μmol/L) diluted in 0.2 mL of RPMI 1640 supplemented with 100 mL/L FCS were added. After incubation for 48 h, 20 μL of 5 mg/mL MTT was added and the plate was incubated for a further 4 h before the medium was discarded. Then 150 μL DMSO was added to dissolve the formazan. Absorbence values (A) were measured at a wavelength of 490 nm with a microplate spectrophotometer. Values were expressed as mean±SD of 12 wells and surviving rate was calculated as follows: Surviving rate = A490 of experiment /A490 of control×100%. All determinations were carried out in triplicate.
Approximately 1.0×107 HCT116 cells in 0.2 mL serum-free medium were implanted subcutaneously into the right flank of each mouse. Tumors were allowed to grow up to 4-5 mm in diameter and then the mice were randomized into two groups (five mice per group) to receive daily administration of drug vehicle (5 mL/L DMSO) or indomethacin (3 mg/kg in 5 mL/L DMSO) by gavage. Mouse weight and tumor size were measured every other day. The tumor volume was determined by measuring the longest diameter (a) and the shortest diameter (b) of implanted tumors and calculated according to the formula[14]: Volume (mm3) = 0.5×a×b2. Twenty-seven days after initiation of drug treatment, the mice were sacrificed by cervical dislocation and then the tumors were removed and weighed. The inhibition rate was calculated using the formula: Inhibition rate (%) = (1.0-tumor volume of indomethacin treated group/tumor volume of DMSO-containing group)×100%.
Tissue immunohistochemical staining was performed with the Histostain-SP kit. Tumor tissue samples were fixed in 40 mg/L phosphate-buffered formalin, embedded in paraffin, serially sectioned at a thickness of 4 μm and then deparaffinized. The procedure of immunohistochemical determination was performed according to the manufacturer’s instructions. Monoclonal mouse antibodies against human CD34 and VEGF were used at a dilution of 1:50.
Vessel staining and counting All blood vessels were highlighted by staining endothelial cells for CD34 with a standard immunoperoxidase technique described above. According to Weidner et al[15], any brown-staining endothelial cell or endothelial-cell cluster that was clearly separated from adjacent microvessels, tumor cells, and other connective-tissue elements was considered as a single, countable microvessel. First, three areas of the highest neovascularization (hotspots) were identified by scanning the tumor sections at a low power (10×10). Then, individual microvessels were counted on high power fields (10×40). The average vessel number was calculated.
VEGF staining score Staining of tissue specimens was scored using a semiquantitative scoring system as described by Bresalier et al[16]. Using the 10×objective, staining intensity and distribution in each field was scored as absent (0), weak (1), moderate (2) or strong (3). All 10×fields in a given specimen were individually scored, the percentage of fields at each intensity was determined and scores were added to yield an average staining intensity score (IS) for the entire specimen.
IS = [ (0×F0) +(1×F1)+(2×F2)+(3×F3) ] , F = % 10×fields, where F0 is the percent of 10 fields scored as 0, F1 is the percent scored as 1, F2 is the percent scored as 2, and F3 is the percent scored as 3.
Data was expressed as mean±SD. Statistical analysis, using the Student’s t test and correlated Spearman test, was carried out with the software package SPSS 10.0. P<0.05 was considered statistically significant.
The effect of indomethacin on HCT116 cell viability was assessed quantitatively by MTT assay. Indomethacin, a non-selective COX inhibitor, significantly decreased the viability of HCT116 cells in a dose-dependent manner (Figure 1), with 50% inhibition at approximately 318.2±12.7 μmol/L.
Oral administration of indomethacin (3 mg/kg/d) significantly suppressed tumor growth of the HCT116 xenografts (Figure 2). The tumor volume of treated group (458.89±32.07 mm3) was significantly decreased compared to the control group (828.21±31.59 mm3) (P<0.05). The tumor weight of treated group (0.58±0.12 g) was markedly reduced compared to control group (0.91±0.16 g) (P<0.05). However, no differences were observed in the body weight of the mice between the two groups throughout the study (P>0.05, Table 1) and we did not see any gastrointestinal complications in the treated group.
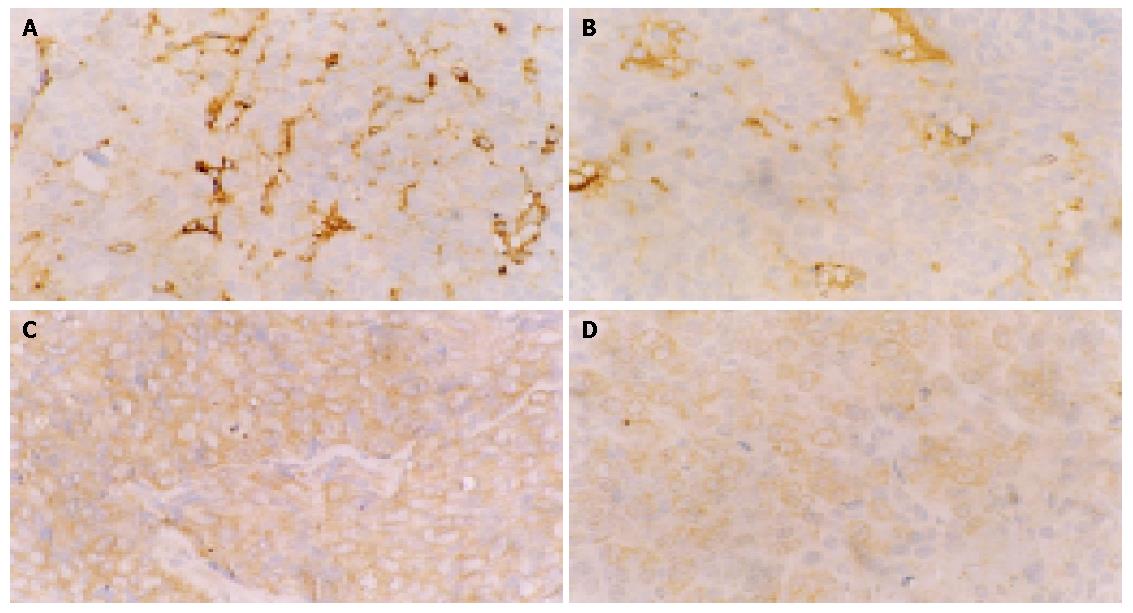
Vascularization was identified by staining the tumors with an antibody to CD34 and the number of vessels per high power field was scored. These areas of high neovascularization could occur anywhere in the tumor, but were most frequent at the margins of carcinomas. Indomethacin significantly reduced tumor angiogenesis relative to controls. The MVD of the treated group (19.50±5.32) was markedly decreased compared to the control group (37.40±4.93) (P<0.001). VEGF expression was mainly localized in cytoplasm of tumor cells. The VEGF expression of the treated group (1.19±0.17) was obviously reduced as compared with the control group (1.90±0.48) (P<0.01). The decrease in MVD was positively correlated with the decrease of VEGF expression (rs = 0.714, P<0.05) (Table 2). Representative sections from control and indomethacin treated tumors are shown in Figure 3.
It is generally accepted that solid tumor growth and metastasis are dependent upon the acquisition of adequate blood supply. Pharmacological targeting of the microvasculature in patients with cancer represents an attractive therapeutic approach because inhibition of angiogenesis has been shown to prevent tumor growth[17], and induced regression of experimental solid tumors[18].
Based on the experimental data and the literature[19], the mechanisms by which NSAIDs inhibit angiogenesis appear to be multifactorial and likely include local changes in angiogenic growth factor expression, alteration in key regulators and mediators of VEGF, increased endothelial cell apoptosis, inhibition of endothelial cell migration, recruitment of inflammatory cells and platelets, and/or thromboxane A2 mediated effects.
VEGF is one of the most potent growth factors for endothelial cells and is involved in physiological and pathological angiogenesis. VEGF mediates its biological effects on the vascular endothelium mainly by binding to two tyrosine-kinase III receptors VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1) and by activating different pathways and enzymes, including mitogen-activated protein kinase (MAPK), stress-activated protein kinase (SAPK), p13-kinase/Akt (serine threonine kinase) and p38 MAPK, which underlie the proliferation, migration and cell survival of endothelial cells and vessel formation[20]. Therefore, VEGF is an ideal target for blocking tumor angiogenesis. Our study demonstrated that there was a strong correlation between the inhibitory effect of indomethacin on MVD and VEGF expression, which is consistent with the literature[21-23]. COX-2 is considered to be a critical mediator of tumor angiogenesis. Mice lacking COX-2 are found to have deficient VEGF expression, reduced tumor angiogenesis and decreased tumor growth[24]. COX-2 inhibitors could decrease VEGF production in tumor cells, and prevent VEGF-induced MAPK activation in endothelial cells[2]. On the other hand, COX-1 has been reported to express constitutively in most tissues but at particularly high levels in endothelial cells[25]. Nonselective COX inhibitors (traditional NSAIDs) might inhibit angiogenesis by two mechanisms: inhibition of COX-2 activity in colon carcinoma cells to down-regulate production of angiogenic factors, and inhibition of COX-1 activity in endothelial cells to suppress endothelial tube formation[26].
However, a few gastrointestinal cancers do not express COX. For example, human colon cancer cell line HCT116 expresses neither COX-1 nor COX-2[26]. In our study, indomethacin (nonselective COX-1 and -2 inhibitor)[27,28] significantly decreased the viability of cultured HCT116 cells and retarded the growth of HCT116 xenografts in nude mice. Immunohistochemical staining demonstrated that MVD and VEGF expressions in the treated tumor tissues were remarkably reduced compared to the controls. Furthermore, the decrease in MVD was positively correlated with the down-regulation of VEGF (rs = 0.714, P<0.05). These results suggest that indomethacin can inhibit human colon cancer both in vitro and in vivo, and may exert its anti-tumor effect by suppressing the new vessel formation (the key step for tumor development), and inhibition of angiogenesis by indomethacin is not entirely dependent on COX, but is associated with VEGF expression inhibition.
It has been reported that harmful side effects of indomethacin on gastric mucosa result from inhibition of gastric COX-1[29] and angiogenesis in granulation tissue at the ulcer base[30]. Further studies are needed to clarify the precise mechanisms by which NSAIDs impair physical and pathological angiogenesis, and to improve the safety and efficacy of these drugs as major anti-cancer agents.
Edited by Kamar M and Wang XL
| 1. | Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Kawai N, Tsujii M, Tsuji S. Cyclooxygenases and colon cancer. Prostaglandins Other Lipid Mediat. 2002;68-69:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Rigas B, Shiff SJ. Nonsteroidal anti-inflammatory drugs and the induction of apoptosis in colon cells: evidence for PHS-dependent and PHS-independent mechanisms. Apoptosis. 1999;4:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Smartt HJ, Elder DJ, Hicks DJ, Williams NA, Paraskeva C. Increased NF-kappaB DNA binding but not transcriptional activity during apoptosis induced by the COX-2-selective inhibitor NS-398 in colorectal carcinoma cells. Br J Cancer. 2003;89:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Babbar N, Ignatenko NA, Casero RA, Gerner EW. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278:47762-47775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Falkowski M, Skogstad S, Shahzidi S, Smedsröd B, Sveinbjörnsson B. The effect of cyclooxygenase inhibitor diclofenac on experimental murine colon carcinoma. Anticancer Res. 2003;23:2303-2308. [PubMed] |
| 7. | Huang L, Ouyang Q, Wei D, Liu X, Hu R. The growth inhibition of colorectal adenoma cells by sulindac and its mechanisms. HuaXi YiKe DaXue XueBao. 2002;33:101-103. [PubMed] |
| 8. | Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003;9:383-390. [PubMed] |
| 9. | Kim TI, Jin SH, Kang EH, Shin SK, Choi KY, Kim WH. The role of mitogen-activated protein kinases and their relationship with NF-kappaB and PPARgamma in indomethacin-Induced apoptosis of colon cancer cells. Ann N Y Acad Sci. 2002;973:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Rayyan Y, Williams J, Rigas B. The role of NSAIDs in the prevention of colon cancer. Cancer Invest. 2002;20:1002-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, Tarnawski AS. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 581] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 12. | Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306-1311. [PubMed] |
| 13. | Gately S, Kerbel R. Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis. Prog Exp Tumor Res. 2003;37:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Sawaoka H, Tsuji S, Tsujii M, Gunawan ES, Sasaki Y, Kawano S, Hori M. Cyclooxygenase inhibitors suppress angiogenesis and reduce tumor growth in vivo. Lab Invest. 1999;79:1469-1477. [PubMed] |
| 15. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4014] [Cited by in RCA: 4088] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 16. | Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, Brodt P, Byrd JC. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110:1354-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 135] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Lannutti BJ, Gately ST, Quevedo ME, Soff GA, Paller AS. Human angiostatin inhibits murine hemangioendothelioma tumor growth in vivo. Cancer Res. 1997;57:5277-5280. [PubMed] |
| 18. | O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3111] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 19. | Tarnawski AS, Jones MK. Inhibition of angiogenesis by NSAIDs: molecular mechanisms and clinical implications. J Mol Med (Berl). 2003;81:627-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Yilmaz A, Kliche S, Mayr-Beyrle U, Fellbrich G, Waltenberger J. p38 MAPK inhibition is critically involved in VEGFR-2-mediated endothelial cell survival. Biochem Biophys Res Commun. 2003;306:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Connolly EM, Harmey JH, O'Grady T, Foley D, Roche-Nagle G, Kay E, Bouchier-Hayes DJ. Cyclo-oxygenase inhibition reduces tumour growth and metastasis in an orthotopic model of breast cancer. Br J Cancer. 2002;87:231-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Li G, Yang T, Yan J. Cyclooxygenase-2 increased the angiogenic and metastatic potential of tumor cells. Biochem Biophys Res Commun. 2002;299:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Szabó IL, Pai R, Soreghan B, Jones MK, Baatar D, Kawanaka H, Tarnawski AS. NSAIDs inhibit the activation of egr-1 gene in microvascular endothelial cells. A key to inhibition of angiogenesis? J Physiol Paris. 2001;95:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 524] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 25. | Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2123] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 26. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 1644] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 27. | Gierse JK, Hauser SD, Creely DP, Koboldt C, Rangwala SH, Isakson PC, Seibert K. Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase. Biochem J. 1995;305:479-484. [PubMed] |
| 28. | Wilson JE, Chandrasekharan NV, Westover KD, Eager KB, Simmons DL. Determination of expression of cyclooxygenase-1 and -2 isozymes in canine tissues and their differential sensitivity to nonsteroidal anti-inflammatory drugs. Am J Vet Res. 2004;65:810-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | DeWitt DL, Meade EA, Smith WL. PGH synthase isoenzyme selectivity: the potential for safer nonsteroidal antiinflammatory drugs. Am J Med. 1993;95:40S-44S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Tarnawski A. Molecular mechanisms of ulcer healing. Drug News Perspect. 2000;13:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |