Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4427
Revised: May 8, 2005
Accepted: May 13, 2005
Published online: July 28, 2005
AIM: To examine the effect of prostaglandin E2 (PGE2) on the expression of vascular endothelial growth factor (VEGF) mRNA in the human hepatocellular carcinoma (HCC) HepG2 cells and the possible involvement of c-fos protein in this process.
METHODS: Human HCC HepG2 cells were divided into three groups treated respectively with PGE2, a combination of PGE2 and c-fos antisense oligodeoxynucleotide (ASO), and PGE2 plus c-fos sense oligodeoxynucleotide (SO). The expression of VEGF mRNA in HepG2 cells after different treatments was detected by reverse transcriptase-polymerase chain reaction (RT-PCR). The relative expression level of VEGF mRNA in HepG2 cells in each group was measured.
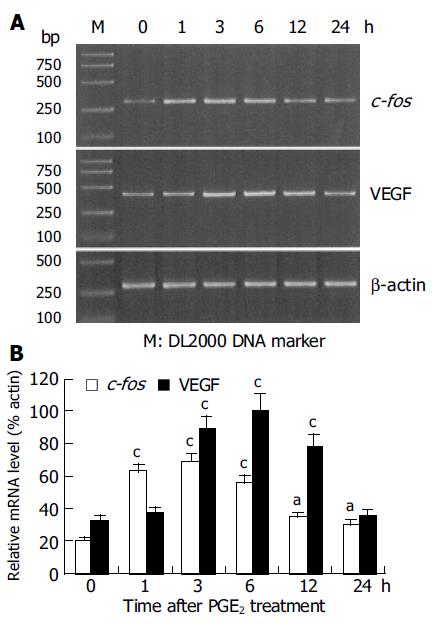
RESULTS: Administration of PGE2 resulted in an increased expression of c-fos and VEGF mRNA in HepG2 cells. The relative expression level of c-fos mRNA reached the peak at 3 h (68.44.7%) after PGE2 treatment, which was significantly higher than that at 0 h (20.6±1.7%, P<0.01). Whereas, the highest expression level of VEGF mRNA was observed at 6 h (100.5±6.1%) after PGE2treatment, which was significantly higher than that at 0 h (33.2±2.4%, P<0.01). C-fos ASO significantly reduced PGE2-induced VEGF mRNA expression in HepG2 cells.
CONCLUSION: PGE2 increases the expression and secretion of VEGF in HCC cells by activating the transcription factor c-fos, promotes the angiogenesis of HCC and plays an important role in the pathogenesis of liver cancer.
- Citation: Li YQ, Tao KS, Ren N, Wang YH. Effect of c-fos antisense probe on prostaglandin E2 -induced upregulation of vascular endothelial growth factor mRNA in human liver cancer cells. World J Gastroenterol 2005; 11(28): 4427-4430
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4427.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4427
PGE2 is produced in various kinds of cancer cells and seems to be particularly important for carcinogenesis[1-3]. PGE2 activates multiple G-protein-linked receptor subtypes (EP1-EP4) in an autocrine or paracrine fashion, which may lead to tumor growth promotion via growth factors and oncogenes[4-6]. However, the mechanism of PGE2 in promoting tumor growth still remains unclear. VEGF is a regulator of pathological angiogenesis and plays an important role in tumor growth. Studies have revealed that VEGF can be produced by liver cancer cells in a paracrine manner, thus promoting the angiogenesis of liver cancer[7,8]. Studies also indicate that many tumor growth factors stimulate the production of VEGF in tumor cells[3,9]. This study was undertaken to estimate if PGE2 could affect the expression of VEGF in HCC HepG2 cells and the possible involvement of the oncogene c-fos in this process.
HepG2 cells were cultured in RPMI-1640 medium (Gibco) containing 10 mL/L fetal bovine serum, 100 kU/L penicillin and 0.1 g/L streptomycin at 37 °C in 50 mL/L CO2/950 mL/L air for 4-6 d and then put into fresh 35 mm dishes. Twenty-four hours later, PGE2(Sigma) was added into each dish in a final concentration of 1 mmol/L. The dose of PGE2 in the present study was chosen based on the previous reports and our preliminary experiments. The cells were then cultured for 0, 1, 3, 6, 12, and 24 h, respectively (n = 4/each time point) and collected for RNA extraction.
C-fos ASO (5'-GAACATCATCGTGGC-3') was synthesized according to reported human c-fos mRNA sequence (GenBank Accession No. M16287). C-fos SO (5'-GCCA-CGATGATGTTC-3') was also synthesized as a control. Both ASO and SO were modified phosphorothioate oligodeoxynucleotide.
HepG2 cells were cultured as mentioned above and divided into: (1) control group in which 10 mL physical saline was added, (2) PGE2-treated groups in which 1 mmol/L of PGE2 was added, (3) SO-treated group in which 10 mL (50 mg) c-fos SO was added followed by addition of 1 mmol/L of PGE2 after 30 min, (4) ASO-treated group in which 10 mL (50 mg) c-fos ASO was added followed by addition of 1 mmol/L of PGE2 after 30 min. The cells were cultured for 6 h and then collected for RNA extraction.
Specific primers for human c-fos and VEGF were synthesized according to their reported mRNA sequences. The primer pair of c-foswere: sense: 5'- TGC TGA AGG AGA AGG AAA AA -3'; antisense: 5'- TGC ATA GAA GGA CCC AGA TA -3' (GenBank Accession No. M16287). The primer pair of VEGF were: sense: 5'-ACC CAT GGC AGA AGG AGG AG -3' antisense 5'-ACG CGA GTC TGT GTT TTT GC-3' (GenBank Accession No. M32977). The primers (sense: 5'- GGC ATC CAC GAA ACT ACC TT-3' antisense 5'-CGT CAT ACT CCT GCT TGC TG -3') for human b-actin (GenBank Accession No. M10277) were also synthesized as internal control in the PCR reaction. The length of PCR product for c-fos, VEGF and b-action was 344 bp, 433 bp and 274 bp, respectively.
Total cellular RNA was extracted from HepG2 cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The purity and integrity of the RNA samples were assessed by A260/280 spectrophotometric measurement.
After measurement of the concentration, cDNA was reversely transcribed in a 50 mL mixture containing 2 mg total RNA, 10 mL 5 RT buffer, 5 mL 10 mmol/L dNTPs, 0.5 mL RNase inhibitor (4×105 U/L, Invitrogen) 0.5 mL oligo (dT)12-18 (500 g/L, Invitrogen) 1 mL SuperScript II reverse transcriptase (2×104 U/L, Invitrogen), 0.5 mL 0.1 mol/L DTT at 42 °C for 60 min followed by enzyme denaturation at 70 °C for 10 min. Thirty cycles of PCR were carried out in 25 mL reaction mixture containing 0.1 mg synthesized cDNA, 2.5 mL 10 PCR buffer, 2.5 mL dNTPs (2 mmol/L), 2.5 mL MgCl2 (2.5 mmol/L), 1 mL of each primer (20 mmol/L), 2.5 u of Taq DNA polymerase (Takara) using a PTC-100 programmed thermal controller (MJ Research), each consisting of denaturation at 94°C for 1 min, annealing at 56 °C for 30 s, extension at 72 °C for 1 min. Then, 10 mL of each PCR product was separated by electrophoresis on a 30 g/L agarose gel and visualized by ethidium bromide staining.
For each template, PCR amplification was performed 2-3 times. The electrophoresis results were observed through a gel imaging system (UVP) and the density of each positive band was analyzed by Labworks software. The relative expression level of c-fos and VEGF mRNA was expressed as a ratio of densitometric measurements (c-fos/b-actin or VEGF/b-actin). The data were expressed as mean±SE, and analyzed by analysis of variance and Dunnets test using SPSS10.1 software.
Addition of PGE2 to the HepG2 cells resulted in a time-dependent increase in the expression of c-fos and VEGF mRNA (Figure 1A). Compared to the expression level at 0 h (20.6±1.7%), the expression of c-fos mRNA induced by PGE2 treatment reached the highest level at 1 h (62.3±4.3%, P<0.01) and 3 h (68.4±4.7%, P<0.01), and slightly higher level at 6 h (55.3±3.8%, P<0.05; Figure 1B). The expression level of VEGF mRNA significantly increased at 3 h after PGE2 administration (87.6±6.4%, P<0.01) when compared to the expression level at 0 h (33.2±2.4%). Its expression level reached a maximum at 6 h (100.5±6.1%, P<0.01). At 24 h, the expression level returned to its level at 0 h (35.2±2.8%, P>0.05; Figure 1B). The expression level of b-actin mRNA remained unchanged at each time-point, indicating the equal amount of the template used in PCR.
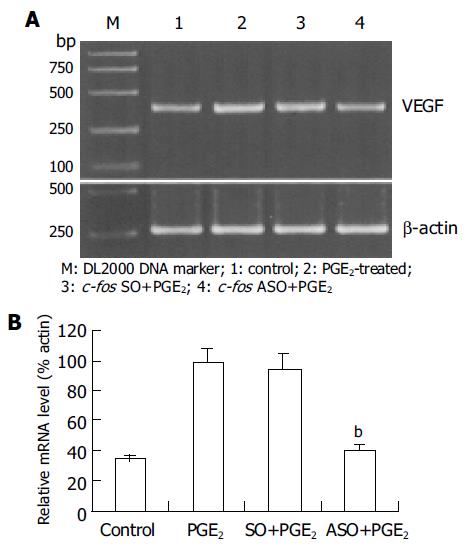
Since the maximal expression level of VEGF mRNA was at 6 h after PGE2 treatment, this time-point was selected to observe the effect of c-fos ASO. The results showed that the expression level of VEGF mRNA significantly decreased in c-fos ASO-treated group (39.6±3.2%) when compared to that in PGE2-treated group (98.6±6.4%, P<0.01, Figure 2A and B). In contrast, no such change in c-fos SO-treated group was observed (95.2±6.3%, P>0.05).
At present, the exact pathological function and mechanism of PGE2 in tumors are not fully known. Previous studies indicate that PGE2 can be produced by tumor cells and plays an important role in tumor immune inhibition[10-12]. Some studies revealed that the PGE2 level in patients with cancer is higher than that in normal people, and that tumor tissues also contain higher concentration of PGE2 than normal tissues[13]. Animal experiments indicate that PGE2 produced by tumor cells, can promote the growth and development of tumors through its immune inhibitory function[10]. Further studies have proved that PGE2 promotes the growth of liver cancer through its receptor EP3[14]. In the present study, we observed that PGE2 could stimulate the expression of VEGF mRNA in HepG2 cells in a time-dependent manner, suggesting that PGE2 may promote the angiogenesis of HCC by increasing the secretion of VEGF from liver cancer cells. This might be one of the mechanisms of PGE2 in facilitating the growth of liver cancer.
It is well known that the oncogene c-fos can function as a third intracellular messenger. Its product Fos protein can form a homo-dimer itself or hetero-dimer with c-Jun protein and then binds to the AP-1 site in the target gene, thus promoting the transcription of target gene. It has been reported that the promoter region for the VEGF gene contains several AP-1 binding motifs[15] and the expression of VEGF gene is controlled by transcription factors AP-1 and AP-2[16-18]. In the present study, we observed that PGE2 increased the expression of c-fos mRNA, the maximal level was at 1 and 3 h after PGE2 administration, earlier than the PGE2-induced highest expression of VEGF mRNA. Furthermore, c-fos ASO significantly reversed PGE2-induced VEGF mRNA expression. These results indicate that Fos protein is involved in the PGE2-induced VEGF expression in HepG2 cells.
The intracellular signaling pathway coupled to PGE2 is complicated. As a third intracellular messenger, c-fos is just located in the downstream of the signaling pathway. Many other molecules should also be involved in the modulation of VEGF expression by PGE2. In addition, several PGE2 receptors are present in HCC[6,19]. Which receptors mediate the role of PGE2 in tumor growth needs to be investigated.
In conclusion, PGE2 stimulates VEGF induction in HepG2 cells by activating the transcription factor Fos protein.
The authors thank Dr. SX Wu, Department of Anatomy, Faculty of Basic Medicine, Fourth Military Medical University for his technical assistance and help with preparation of the manuscript.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Bishop-Bailey D, Calatayud S, Warner TD, Hla T, Mitchell JA. Prostaglandins and the regulation of tumor growth. J Environ Pathol Toxicol Oncol. 2002;21:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Ito H, Duxbury M, Benoit E, Clancy TE, Zinner MJ, Ashley SW, Whang EE. Prostaglandin E2 enhances pancreatic cancer invasiveness through an Ets-1-dependent induction of matrix metalloproteinase-2. Cancer Res. 2004;64:7439-7446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, Chung SC, Sung JJ. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003;23:1317-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Ushio A, Takikawa Y, Lin SD, Miyamoto Y, Suzuki K. Induction of Bcl-xL is a possible mechanism of anti-apoptotic effect by prostaglandin E2 EP4-receptor agonist in human hepatocellular carcinoma HepG2 cells. Hepatol Res. 2004;29:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Yamaki T, Endoh K, Miyahara M, Nagamine I, Thi Thu Huong N, Sakurai H, Pokorny J, Yano T. Prostaglandin E2 activates Src signaling in lung adenocarcinoma cell via EP3. Cancer Lett. 2004;214:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, Kobayashi M, Satoh K, Narita M, Sugimoto Y. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med. 2003;197:221-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Imura S, Miyake H, Izumi K, Tashiro S, Uehara H. Correlation of vascular endothelial cell proliferation with microvessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in hepatocellular carcinoma. J Med Invest. 2004;51:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Casibang M, Purdom S, Jakowlew S, Neckers L, Zia F, Ben-Av P, Hla T, You L, Jablons DM, Moody TW. Prostaglandin E2 and vasoactive intestinal peptide increase vascular endothelial cell growth factor mRNAs in lung cancer cells. Lung Cancer. 2001;31:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 870] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 11. | Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol. 2002;2:603-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Kaur K, Harris SG, Padilla J, Graf BA, Phipps RP. Prostaglandin E2 as a modulator of lymphocyte mediated inflammatory and humoral responses. Adv Exp Med Biol. 1999;469:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Majima M, Amano H, Hayashi I. Prostanoid receptor signaling relevant to tumor growth and angiogenesis. Trends Pharmacol Sci. 2003;24:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Hashimoto N, Watanabe T, Ikeda Y, Yamada H, Taniguchi S, Mitsui H, Kurokawa K. Prostaglandins induce proliferation of rat hepatocytes through a prostaglandin E2 receptor EP3 subtype. Am J Physiol. 1997;272:G597-G604. [PubMed] |
| 15. | Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947-11954. [PubMed] |
| 16. | Clifford SC, Czapla K, Richards FM, O'Donoghue DJ, Maher ER. Hepatocyte growth factor-stimulated renal tubular mitogenesis: effects on expression of c-myc, c-fos, c-met, VEGF and the VHL tumour-suppressor and related genes. Br J Cancer. 1998;77:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Ryuto M, Ono M, Izumi H, Yoshida S, Weich HA, Kohno K, Kuwano M. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220-28228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 329] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995;372:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 376] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Bhattacharya M, Peri K, Ribeiro-da-Silva A, Almazan G, Shichi H, Hou X, Varma DR, Chemtob S. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J Biol Chem. 1999;274:15719-15724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |