Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4375
Revised: January 2, 2005
Accepted: January 5, 2005
Published online: July 28, 2005
AIM: Delayed gastric emptying and an enlarged fasting gastric antrum are common findings in functional dyspepsia but their relationship with gastrointestinal (GI), and the frequently associated extra-GI symptoms remains unclear. This study evaluated the relationship between GI and extra-GI symptoms, fasting antral volume and delayed gastric emptying in functional dyspepsia.
METHODS: In 108 functional dyspeptic patients antral volume and gastric emptying were assessed with ultraso-nography (US). Symptoms were assessed with standardized questionnaire. The association of symptoms and fasting antral volume with delayed gastric emptying was estimated with logistic regression analysis.
RESULTS: Delayed gastric emptying was detected in 39.8% of the patients. Postprandial drowsiness (AOR 11.25; 95%CI 2.75-45.93), nausea (AOR 3.51; 95%CI 1.19-10.32), fasting antral volume (AOR 1.93; 95%CI 1.22-3.05), were significantly associated with delayed gastric emptying. Symptoms, mainly the extra-GI ones as postprandial drowsiness and nausea, combined with fasting antral volume predicted the modality of gastric emptying with a sensitivity and specificity of 78%.
CONCLUSION: In functional dyspeptic patients, (1) an analysis of fasting antral volume and of symptoms can offer valuable indication on the modality of gastric emptying, and (2) it seems appropriate to inquire on postprandial drowsiness that showed the best correlation with delayed gastric emptying.
- Citation: Pallotta N, Pezzotti P, Calabrese E, Baccini F, Corazziari E. Relationship between gastrointestinal and extra-gastrointestinal symptoms and delayed gastric emptying in functional dyspeptic patients. World J Gastroenterol 2005; 11(28): 4375-4381
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4375
The term dyspepsia is widely used in clinical practice to describe symptoms arising from the upper abdomen and, depending on the definition, its prevalence has been reported to vary from 20% to 40%[1-3] in the adult population. Although dyspeptic symptoms may arise from several pathological conditions, more than 70%[4-8] of patients, do not have definite structural or biochemical alterations[9-11] and, based on the Rome Diagnostic Criteria functional dyspepsia (FD) is made[12,13]. In the assumption that symptoms may predict specific underlying pathophysiology of FD it has been suggested that patients be subdivided in accordance with symptom clusters[12] such as ulcer-like and dysmotility-like dyspepsia or, more recently, with the predominant symptom[13]. Several studies have looked for possible correlation between symptoms and pathophysiological abnormalities such as Helicobacter pylori infection, visceral hypersensitivity and abnormal motor function of the stomach. Up to 40% of patients with FD evaluated in referral centers have delayed gastric emptying[14] and 40% impaired postprandial relaxation of the proximal stomach[15]. So far however, there is little evidence[16] that gastric motor abnormalities correlate unequivocally with different symptom clusters[17-19]and not even in those patients with dysmotility-like dyspepsia, referring symptoms suggestive of an abnormal motor function of the stomach such as postprandial fullness, nausea, early satiety and vomiting. Female gender, and, severe and predominant, postprandial fullness and vomiting have been reported to be associated with delayed gastric emptying[20], a finding not confirmed in a subsequent study[21] performed in a large cohort of patients with dysmotility-functional and organic (i.e., diabetic) dyspepsia. Drowsiness is a subjective experience often reported after food ingestion and it has been shown that solid meal results in a decreased sleep onset latency in healthy volunteers[22].
Patients with functional dyspepsia complain of several gastrointestinal (GI) and extra-GI symptoms[23], some of the latter like drowsiness[24,25] and headache[24] are related to meal ingestion. It is not known, however, whether post-prandial drowsiness and other extra-GI symptoms have any relationship with gastric functions.
An enlarged fasting antral volume assessed by ultrasonography (US)[26,27] is an additional finding in patients with FD but its relationship, if any, with delayed gastric emptying is not known.
We, therefore aimed to evaluate in functional dyspeptic patients whether and which symptoms either GI or extra-GI are related to, and might predict, delayed gastric emptying of a regular meal. Additional aim was to assess whether the US measurement of basal antral volume may predict the modality of gastric emptying.
Two hundred and ten consecutive patients referring symptoms of dyspepsia (140 F; age 42.8±12.5 years, mean±SD) referred to the gastroenterology outpatient clinic were assessed. Functional dyspepsia was defined as persistent or recurrent pain or discomfort centered in the upper abdomen for at least 3 mo in the preceding 12 mo, in the absence of any known organic disease that is likely to explain the symptoms[12,13] and no evidence that dyspeptic symptoms were exclusively relieved by defecation or associated with the onset of a change in stool frequency or stool form[13].
Organic abnormalities, psychiatric illnesses, history of alcohol abuse, use of NSAIDs, steroids or drugs affecting gastric functions, previous surgery of the GI tract (except appendectomy and cholecystectomy) and systemic disorders were ruled out by history, clinical examination, biochemical investigations, upper GI endoscopy, and transabdominal US. Dyspeptic patients with Rome diagnostic criteria of irritable bowel syndrome (IBS) and/or referring heartburn and/or regurgitation as predominant or frequent symptoms, were excluded from the study.
Epigastric pain and upper abdominal discomfort were graded 0-4 according to its influence on patient's daily activities: 0, absent; 1, mild (present but easily bearable if distracted by usual activities); 2, moderate (bearable but not influencing usual activities); 3, relevant (influencing usual activities); 4, severe (interruption of usual activities).
Patients were enrolled into the study if symptomatic at the time of evaluation with a symptom score value of ≥2 for epigastric pain or discomfort.
Overall 102 patients (67 F, age 46±13.2 years, mean±SD) were excluded from the study because of the following diagnosis: gastroesophageal reflux disease, 52; IBS, 15; peptic ulcer, 14; migraine, 16; psychiatric disorders, 3; celiac disease, 2.
One hundred and eight patients with functional dyspepsia entered the study (73 F, age 42±12.5 years, mean±SD). Twenty-eight healthy asymptomatic volunteers (10 F, mean age 31±5.5 years, mean±SD) were also investigated. None of them had GI disorders or symptoms or were taking medications of any kind. None had been previously submitted to surgery of the GI tract.
Informed consent was obtained from each subject and the study protocol was approved by the local ethics committee.
Consecutive patients were interviewed with a standardized questionnaire[28] made of 50 items, inquiring on demography (5 items), daily habits (10 items) that included meal timing and composition, alcohol consumption, smoking and sleep, past medical history (3 items), GI symptoms (15 items), gastroesophageal symptoms (4 items), bowel pattern (7 items), and somatic extra-GI symptoms that included 5 items as indicated in a previously published study[23]. Postprandial drowsiness, defined as a state of impaired awareness associated with a desire or inclination to sleep[29] was also included in the questionnaire since this meal-related symptom has been reported[2] and confirmed by personal observations, to be bothersome in dyspeptic patients. Dyspeptic symptoms were defined according to Rome criteria[12,13].
Frequency and time relationship with meal assumption was assessed for each investigated symptom and, relationship of drowsiness with sleep disturbances was specifically looked for.
In addition patients were requested to refer any other symptom they considered to be bothersome and relating to meal ingestion.
Gastric emptying was evaluated with US according to previously validated and standardized methods[30-34]. Gastric antral volume was evaluated by US according to previously published methods[33,34] with a 4 MHz linear probe and 3.5 MHz convex probe (Toshiba SAL 38B, Tosbee, Toshiba, Japan).
All drugs affecting the GI tract were discontinued at least 3 d before the gastric emptying studies. Subjects refrained from smoking for a 12-h period preceding, and during, the examination. After an overnight fast the subjects ate an ordinary standard solid 1 050 kcal meal containing 140 g bread, 70 g cheese, 80 g ham, (50% carbohydrates, 25% lipids, 25% proteins), 3.5 g alimentary fibers, 250 mL of water. The time of meal ingestion did not exceed 30 min (range 15-30 min). Gastric antral US measurements were performed by the same operator with the subjects standing in the upright position, in fasting condition, immediately, and at 30 and 60 min after the end of the meal ingestion, and at 60-min intervals thereafter over a total period of 300 min. In the intervals between measurements subjects could move freely.
Delayed gastric emptying was defined as the final antral volume (i.e., gastric antral volume at 300 min after meal ingestion) exceeding the mean value plus 2SDs of the 28 healthy volunteers (31 mL).
Descriptive statistics as median values and interquartile ranges were calculated. Box-plots[35] were used for inter-group comparison of antral volume distributions. The Mann-Whitney test[35] was used to compare the median values of antral volume between healthy controls, FD patients with delayed and those with normal gastric emptying.
Sensitivity and specificity of each symptom for three different threshold levels of fasting antral volume were calculated. Odds ratios (OR) were also calculated as a synthetic measure of both sensitivity and specificity[35].
Logistic regression analysis[36] was then applied to estimate crude and adjusted odds-ratios (AOR) and 95% confidence intervals (95%CI) of having delayed gastric emptying for each symptom, fasting antral volume, gender, age, and body mass index (BMI). In addition, we reported results from two multivariate logistic regression analyses, respectively, without and with fasting antral volume, obtained through a backward selection strategy having excluded factors with a log-likelihood P-value >0.20[35]. Two-sided Pvalues were defined statistically significant when P<0.05, and marginally significant when 0.05<P<0.2.
Estimated coefficients from multivariate logistic regression were used to calculate the probability of having (or not having) delayed gastric emptying. Through these probabilities, it is possible to calculate sensitivity, specificity, and the percentage of patients correctly classified[36]. All the analyses were performed using STATA release 5.0[37].
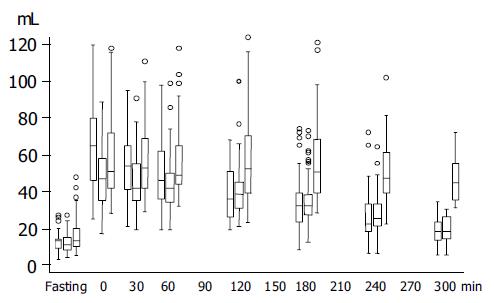
Figure 1 shows antral volume values before, immediately, and in the 300 min after meal ingestion in normal controls and in functional dyspeptic patients with normal and delayed gastric emptying.
In healthy volunteers, the mean gastric antral volume was 135 mL in the fasting state and reached its maximal value at the end of meal ingestion (67±24 mL), then decreased almost linearly to reach 18±6.5 mL at the end of the study. In FD patients, the mean fasting antral volume was 14±8 mL (ns vs controls) and reached its maximal value at the end of the meal (53±21 mL) (ns vscontrols), then decreased to reach the final value of 30±16 mL (P<0.0001 vs controls).
Gastric emptying was delayed in 43 (39.8%) patients (28 F; mean age 41.4 years; range 23-64 years) and their final AV was 46±11 mL (Figure 1). The mean fasting AV (17±10 mL) was larger in patients with delayed gastric emptying than in those with normal gastric emptying (12±5 mL, P = 0.005, Figure 1). The mean delta variation between fasting and final antral volume was 29±14 mL in dyspeptic patients with delayed gastric emptying and 7±8 mL in those with normal gastric emptying (P<0.0001).
Frequency, as well as sensitivity, specificity, and crude OR in predicting delayed gastric emptying of each of the GI and extra-GI symptoms reported by at least eight patients as well as fasting antral volume are reported in Table 1. Delayed gastric emptying was not related to age (OR = 1.03; P = 0.84), gender (OR = 0.7; P = 0.4), while there was a marginally significant inverse association, with BMI (OR = 0.89 per 1 kg/m2 increase; P = 0.06, data not shown in Table 1).
| Prevalence % | Sensitivity % | Specificity % | OR | |
| Pain centered in the upper abdomen | 45.4 | 58.1 | 63.1 | 2.37 |
| Discomfort centered in the upper abdomen | 79.6 | 81.4 | 21.5 | 1.2 |
| Upper abdominal bloating | 84.3 | 88.4 | 18.5 | 1.72 |
| Fullness | 68.5 | 62.8 | 27.7 | 0.64 |
| Early satiety | 45.4 | 48.8 | 56.9 | 1.26 |
| Nausea | 42.6 | 53.5 | 64.6 | 2.1 |
| Heartburn | 26.9 | 34.9 | 78.5 | 1.95 |
| Epigastric burning | 11.1 | 9.3 | 87.7 | 0.73 |
| Belching | 74 | 74.4 | 26.1 | 1.03 |
| Vomiting | 27.8 | 20.9 | 67.7 | 0.55 |
| Acid regurgitation | 19.4 | 23.3 | 83 | 1.48 |
| Drowsiness | 16.7 | 32.6 | 93.9 | 7.36 |
| Headache | 41.7 | 46.5 | 61.5 | 1.39 |
| Palpitation | 7.4 | 6.9 | 92.3 | 0.9 |
| Fasting antral volume (mL) | ||||
| <10 mL (reference group) | 33.3 | 18.6 | 43 | 1.09 |
| 10–14 mL | 34.3 | 39.5 | 30.8 | 2.97 |
| >15 mL | 32.4 | 41.9 | 26.2 | 3.71 |
None of the patients with post-prandial drowsiness complained of sleeplessness.
The AOR for symptoms with or without fasting antral volume and BMI included in the multivariate analyses are shown in Table 2. Not taking into consideration fasting antral volume and BMI in the multivariate analyses, upper abdominal pain, nausea, and drowsiness showed a statistically significant association, whereas upper discomfort showed only a marginally significant association with delayed gastric emptying. Post-prandial fullness or vomiting showed a marginally significant association with normal gastric emptying. Including fasting antral volume and BMI in the multivariate analyses, nausea and drowsiness were highly significantly associated with delayed gastric emptying, while upper abdominal pain, upper abdominal discomfort, and heartburn were only marginally significantly associated with delayed gastric emptying. Post-prandial fullness showed a highly significant, and vomiting a marginally significantly, association with normal gastric emptying. Fasting antral volume was significantly associated with delayed gastric emptying increasing the OR of 93% for any additional volume increase of 5 mL. BMI showed a marginally significant inverse association with delayed gastric emptying with an OR decrease of 11% for any 1 kg/m2 increase.
| Without FAV and BMI | With FAV and BMI | |||
| AOR | 95%CI | AOR | 95%CI | |
| Pain centered in the upper abdomen | 3.74 | 1.34-10.41 | 2.78 | 0.92-8.41 |
| Nausea | 2.92 | 1.15-7.44 | 3.51 | 1.19-10.32 |
| Postprandial fullness | 0.41 | 0.14-1.14 | 0.27 | 0.08-0.84 |
| Discomfort centered in the upper abdomen | 3.42 | 0.88-13.25 | 4.59 | 0.99-21.20 |
| Vomiting | 0.44 | 0.15-1.31 | 0.32 | 0.09-1.11 |
| Heartburn | - | - | 2.31 | 0.80-6.71 |
| Drowsiness | 9.37 | 2.47-35.45 | 11.25 | 2.75-45.93 |
| BMI | - | - | 0.89 | 0.77-1.02 |
| Fasting antral volume1 | - | - | 1.93 | 1.22-3.05 |
Using other two different cut-points (29 and 33 mL), instead of 31 mL, as discriminant final antral volume for normal and delayed gastric emptying, all the results of univariate and multivariate analyses did not vary.
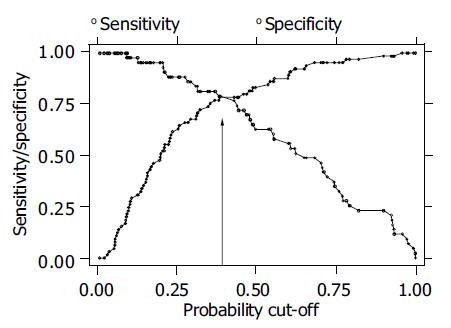
Furthermore probabilities of having delayed gastric emptying were assessed with the selected symptoms, fasting antral volume, and BMI as reported from the second model (Table 2 and Figure 2). Sensitivity and specificity of the applied model are plotted for different values of estimated prevalence of delayed gastric emptying ranging from 0 to 1. At the cut-off value of 0.398, i.e., the estimated prevalence of delayed gastric emptying in this sample, sensitivity and specificity were about 78% with a correct classification of 73.3% of the patients. Applying a model based on selected symptoms only, the estimated sensitivity and specificity were negligibly lower, than those obtained with the model including fasting antral volume and BMI, with a correct classification of 68.1% of the patients at the cut-off value of 0.398 (data not shown).
In the attempt to clarify the pathophysiology of symptoms in functional dyspepsia it has been proposed to classify patients with functional dyspepsia into clinically distinct subgroups on the basis of symptom clusters[12]. Thus the presence of the variable combination of upper abdominal bloating, upper abdominal fullness, early satiety, nausea, belching, retching, and vomiting have been regarded as suggestive of impaired gastric motor activity[38-40]. However several studies[16-19] have so far failed to demonstrate in this subgroup of patients a close correlation between symptoms and disturbances of motor function. More recently[13] it has been proposed to classify dyspeptic subgroup on the basis of the predominant symptom. Nevertheless two studies[15,20] in the attempt to assess the relationship between predominant symptoms and gastric dysfunction reported non-univocal results in populations that did not exclude patients with IBS and gastroesophageal reflux disease (GERD). Delayed gastric emptying was independently associated with severe post-prandial fullness and severe vomiting in the first study[20] and with severe nausea in the second one[15] that also reported early satiety as specifically associated with impaired gastric accommodation to a meal. In both studies, however, the association between the mentioned symptoms and the altered gastric function was not a universal finding but limited to a subgroup of patients. The uncertainty of the matter is further illustrated by a third study[21] that failed to find an association between any of the dyspeptic symptoms, as well as their severity, and delayed gastric emptying.
Although reported in previous studies[23-25] and frequently referred by dyspeptic patients little attention has been paid to other symptoms not referable to the GI tract. The present study aimed to assess whether single GI or extra-GI symptoms and the simple non-invasive US measure of the gastric antral volume in fasting condition could predict the presence of delayed gastric emptying, in a group of dyspeptic patients in whom symptoms of IBS and GERD were excluded.
The present study differs from the previous ones also for other aspects. A meal having the same composition of a normal everyday lunch was used in the present study whereas unusual experimental and/or low caloric meals (≤700 kcal)[17-19] used previously might have not sufficiently challenged the upper GI function. Most of the previously published studies evaluated gastric emptying with the scintigraphic technique that, differently from the US technique, assesses the emptying of the radiolabeled component of the meal rather than of the entire postcibal gastric contents. In addition previous scintigraphic studies have assessed the gastric emptying rate or t1/2 gastric emptying time extrapolated from a limited observation period after meal ingestion, two variables that had been shown to be unable to express final gastric emptying time in functional dyspeptic patients[41]. The serial US measurements of the antral volume enables to assess two relevant aspects of the gastric function, i.e., the antral volume, which is the expression of antral distension and the final gastric emptying time. The former cannot be assessed with scintigraphy and the latter has been shown to be the variable that best correlates with the scintigraphic method and to discriminate patients with delayed gastric emptying from controls[30-32,42]. In the present study extra-GI symptoms were reported by 53.7% of the patients. Of relevance is that of all investigated symptoms, including GI symptoms usually regarded characteristic of dyspepsia, post-prandial drowsiness is the one that most correlates with, and is the best predictor of, delayed gastric emptying. Also nausea and pain centered in the upper abdomen, albeit to a lesser degree than post-prandial drowsiness, showed a statistical correlation with delayed gastric emptying. Differently from typical abdominal dyspeptic symptoms, drowsiness showed a high specificity in predicting delayed gastric emptying. The other most frequently referred extra-GI symptoms such as headache and palpitation were not statistically related with delayed gastric emptying. Drowsiness can occur in several neurological conditions, including autonomic failure and truncal vagotomy. In none of the investigated patients drowsiness could be explained with any detectable disorders. In healthy subjects it has been shown that in comparison with an equal volume of water and equicaloric liquid meal, solid meal results in a decreased sleep onset latencies[22]. Furthermore it has been reported a transient decrease in sleep latency after consuming a meal compared to sham feeding[43]. These results together with the observation of a increased feeling of drowsiness after intravenous injection of CCK administration[44], support the hypothesis of a GI effect on postprandial sleepiness. It is conceivable that meal ingestion may release CCK and other neuroendocrine substances, such as serotonin, or activate nervous afferences, that affect the state of consciousness and delay gastric emptying. Alternatively prolonged postprandial antral distension may participate via vagal afferences in the activation of central nervous network regulating the state of consciousness. The sensation of drowsiness may vary from slight to severe and be related to sleep disturbances. Postprandial drowsiness reported in the present investigation refers to a sensation regarded to be bothersome enough to interfere with the daily activities and it was not related to sleeplessness.
In addition this study shows that the fasting antral volume of 15 mL is the cut-off value that may be used to predict delayed gastric emptying. Adjusting data for fasting antral volume and BMI, the following symptoms: nausea and drowsiness appear to be significant independent predictors of delayed gastric emptying.
None of the symptoms conventionally considered to be manifestation of dyspepsia was of value to discriminate between patients with normal and delayed gastric emptying.
The different results of the present study from previously mentioned scintigraphy-based studies, may be due to the use of a different technique and/or the different selection of the patients and/or the different assessment of patient's symptoms[20].
Alternatively, the different results may express a different underlying dysfunction for some of the dyspeptic symptoms. So it may be hypothesized that in patients with functional dyspepsia early satiety[15] is mainly related to impaired relaxation of the proximal stomach; postprandial fullness and vomiting to a reduced rate of gastric emptying[20]; post-prandial drowsiness, nausea, to a delayed final gastric emptying.
This study confirms[26,27] the frequent occurrence in patients with FD of an abnormally distended antral volume during fasting. Whether increased antral volumes may reflect hypotonia of the antral muscular wall or intraluminal distension secondary to gastric retention could not be addressed in the present study.
An increased final antral volume does not necessarily indicate delayed gastric emptying as it may coexist with a normal gastric emptying rate if the fasting antral volume is increased. In the present study, however, the mean final antral volume of dyspeptic patients with delayed gastric emptying exceeded the fasting antral volume of 28 mL indicating the presence of a genuine slowing of the gastric emptying rate.
To identify the cut-off level of 31 mL to discriminate normal and delayed gastric emptying we used data from a control group of healthy people. Similar results were obtained with additional assessments based on other cut-off levels chosen below and above 31 mL. Compared to our patients the control group had a lower percentage of women and a younger age. However, these differences did not affect the results because these two factors (age and gender) did not have, in the multivariate analysis, any relationship with the gastric emptying. This study confirms previous observations of a statistical correlation between BMI and delayed gastric emptying[20]. This relationship may be interpreted as an effect of gastric dysfunction often associated with symptoms, such as nausea or pain that limit food ingestion. Patients with eating disorders may be underweight and show abnormal gastric function. In the present study patients with such disorders were excluded and the finding of equally delayed gastric emptying in both genders would support that eating disorders were not present. We did not routinely test for H pylori in this study, but there is no evidence that Hp infection has any relationship with symptoms and delayed gastric emptying in functional dyspepsia[45-49]. Thus, it seems unlikely that knowledge of the Hp status would have altered the conclusions of the present study.
In conclusion, the presence or, alternatively, the absence of nausea and post-prandial drowsiness, appear to be indicative of delayed or, respectively, normal gastric emptying in functional dyspeptic patients.
Interestingly, despite that the symptom of post-prandial drowsiness is often reported by dyspeptic patients and in some of them it may be the predominant disturbance, it has not been considered part of the definition of dyspepsia. This study shows that post-prandial drowsiness is the symptom that most correlates with delayed gastric emptying and would suggest including it in the clinical definition of functional dyspepsia.
Although it would appear that in FD patients an analysis of dyspeptic symptoms and fasting antral volume can offer valuable indication on the modality of gastric emptying, their ability, either alone or in combination, of correctly classifying individuals with or without delayed gastric emptying was not greater than 73%.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Johnsen R, Straume B, Førde OH. Peptic ulcer and non-ulcer dyspepsia--a disease and a disorder. Scand J Prim Health Care. 1988;6:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Jones RH, Lydeard SE, Hobbs FD, Kenkre JE, Williams EI, Jones SJ, Repper JA, Caldow JL, Dunwoodie WM, Bottomley JM. Dyspepsia in England and Scotland. Gut. 1990;31:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 279] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259-1268. [PubMed] |
| 4. | Barnes RJ, Gear MW, Nicol A, Dew AB. Study of dyspepsia in a general practice as assessed by endoscopy and radiology. Br Med J. 1974;4:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Möllmann KM, Bonnevie O, Gudbrand Höyer E, Wulff HR. A diagnostic study of patients with upper abdominal pain. Scand J Gastroenterol. 1975;10:805-809. [PubMed] |
| 6. | Horrocks JC, De Dombal FT. Clinical presentation of patients with "dyspepsia". Detailed symptomatic study of 360 patients. Gut. 1978;19:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Holdstock G, Harman M, Machin D, Patel C, Lloyd RS. Prospective testing of a scoring system designed to improve case selection for upper gastrointestinal investigation. Gastroenterology. 1986;90:1164-1169. [PubMed] |
| 8. | Capurso L, Koch M, Dezi A. Towards a quantitative diagnosis of dyspepsia: the value of clinical symptoms. The dyspepsia project report. Ital J Gastroenterol. 1988;20:191-202. |
| 9. | Richter JE. Dyspepsia: organic causes and differential characteristics from functional dyspepsia. Scand J Gastroenterol Suppl. 1991;182:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Heikkinen M, Pikkarainen P, Takala J, Räsänen H, Julkunen R. Etiology of dyspepsia: four hundred unselected consecutive patients in general practice. Scand J Gastroenterol. 1995;30:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Klauser AG, Voderholzer WA, Knesewitsch PA, Schindlbeck NE, Müller-Lissner SA. What is behind dyspepsia? Dig Dis Sci. 1993;38:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Talley NJ, Colin-Jones D, Koch KL. Functional dyspepsia: a classification with guidelines for diagnosis and management. Gastroenterol Int. 1991;4:145-160. |
| 13. | Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45 Suppl 2:II37-II42. [PubMed] |
| 14. | Bytzer P, Talley NJ. Dyspepsia. Ann Intern Med. 2001;134:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 762] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 16. | Barbara L, Camilleri M, Corinaldesi R, Crean GP, Heading RC, Johnson AG, Malagelada JR, Stanghellini V, Wienbeck M. Definition and investigation of dyspepsia. Consensus of an international ad hoc working party. Dig Dis Sci. 1989;34:1272-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 101] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Wegener M, Börsch G, Schaffstein J, Reuter C, Leverkus F. Frequency of idiopathic gastric stasis and intestinal transit disorders in essential dyspepsia. J Clin Gastroenterol. 1989;11:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Waldron B, Cullen PT, Kumar R, Smith D, Jankowski J, Hopwood D, Sutton D, Kennedy N, Campbell FC. Evidence for hypomotility in non-ulcer dyspepsia: a prospective multifactorial study. Gut. 1991;32:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Talley NJ, Shuter B, McCrudden G, Jones M, Hoschl R, Piper DW. Lack of association between gastric emptying of solids and symptoms in nonulcer dyspepsia. J Clin Gastroenterol. 1989;11:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, Marengo M, Corinaldesi R. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 453] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Orr WC, Shadid G, Harnish MJ, Elsenbruch S. Meal composition and its effect on postprandial sleepiness. Physiol Behav. 1997;62:709-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Talley NJ, Phillips SF, Bruce B, Zinsmeister AR, Wiltgen C, Melton LJ. Multisystem complaints in patients with irritable bowel syndrome and functional dyspepsia. Eur J Gastroenterol Hepatol. 1991;3:71-77. |
| 24. | Corinaldesi R, Stanghellini V, Raiti C, Rea E, Salgemini R, Barbara L. Effect of chronic administration of cisapride on gastric emptying of a solid meal and on dyspeptic symptoms in patients with idiopathic gastroparesis. Gut. 1987;28:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 138] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Distrutti E, Fiorucci S, Hauer SK, Pensi MO, Vanasia M, Morelli A. Effect of acute and chronic levosulpiride administration on gastric tone and perception in functional dyspepsia. Aliment Pharmacol Ther. 2002;16:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Ricci R, Bontempo I, La Bella A, De Tschudy A, Corazzieri E. Dyspeptic symptoms and gastric antrum distribution. An ultrasonographic study. Ital J Gastroenterol. 1987;19:215-217. |
| 27. | Hausken T, Berstad A. Wide gastric antrum in patients with non-ulcer dyspepsia. Effect of cisapride. Scand J Gastroenterol. 1992;27:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Talley NJ. Optimal design of treatment trials. In DA Drossman, JE Richter, NJ Talley, WG Thompson, E Corazziari, WE Whitehead (eds). Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology and Treatment. Boston, Little Brown and Company 1994; 265-310. |
| 30. | Hveem K, Jones KL, Chatterton BE, Horowitz M. Scintigraphic measurement of gastric emptying and ultrasonographic assessment of antral area: relation to appetite. Gut. 1996;38:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Benini L, Sembenini C, Heading RC, Giorgetti PG, Montemezzi S, Zamboni M, Di Benedetto P, Brighenti F, Vantini I. Simultaneous measurement of gastric emptying of a solid meal by ultrasound and by scintigraphy. Am J Gastroenterol. 1999;94:2861-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Benini L, Sembenini C, Castellani G, Caliari S, Fioretta A, Vantini I. Gastric emptying and dyspeptic symptoms in patients with gastroesophageal reflux. Am J Gastroenterol. 1996;91:1351-1354. [PubMed] |
| 33. | Bolondi L, Bortolotti M, Santi V, Calletti T, Gaiani S, Labò G. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology. 1985;89:752-759. [PubMed] |
| 34. | Ricci R, Bontempo I, Corazziari E, La Bella A, Torsoli A. Real time ultrasonography of the gastric antrum. Gut. 1993;34:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Altman DG. Practical statistics for medical research. Chapman and Hall;. 1991;. |
| 36. | Hosmer DW, Lemeshow S. Applied logistic regression. New York John Wiley and sons, Inc.;. 1989;. |
| 37. | StataCorp . Stata Statistical Software: Release 5.0. Stata Corporation, College Station, TX, USA,. 1989;. |
| 38. | Malagelada JR, Stanghellini V. Manometric evaluation of functional upper gut symptoms. Gastroenterology. 1985;88:1223-1231. [PubMed] |
| 39. | Rees WD, Miller LJ, Malagelada JR. Dyspepsia, antral motor dysfunction, and gastric stasis of solids. Gastroenterology. 1980;78:360-365. [PubMed] |
| 40. | Metcalf R, Youngs GR. Prevalence of symptoms of dyspepsia. BMJ. 1989;298:526-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Guo JP, Maurer AH, Fisher RS, Parkman HP. Extending gastric emptying scintigraphy from two to four hours detects more patients with gastroparesis. Dig Dis Sci. 2001;46:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Bortolotti M, Bolondi L, Santi V, Sarti P, Brunelli F, Barbara L. Patterns of gastric emptying in dysmotility-like dyspepsia. Scand J Gastroenterol. 1995;30:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Harnish MJ, Greenleaf SR, Orr WC. A comparison of feeding to cephalic stimulation on postprandial sleepiness. Physiol Behav. 1998;64:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Stacher G, Bauer H, Steinringer H. Cholecystokinin decreases appetite and activation evoked by stimuli arising from the preparation of a meal in man. Physiol Behav. 1979;23:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 97] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | McColl K, Murray L, El-Omar E, Dickson A, El-Nujumi A, Wirz A, Kelman A, Penny C, Knill-Jones R, Hilditch T. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med. 1998;339:1869-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 297] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 46. | Blum AL, Talley NJ, O'Moráin C, van Zanten SV, Labenz J, Stolte M, Louw JA, Stubberöd A, Theodórs A, Sundin M. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. Omeprazole plus Clarithromycin and Amoxicillin Effect One Year after Treatment (OCAY) Study Group. N Engl J Med. 1998;339:1875-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 281] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 47. | Talley NJ, Vakil N, Ballard ED, Fennerty MB. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N Engl J Med. 1999;341:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Tucci A, Corinaldesi R, Stanghellini V, Tosetti C, Di Febo G, Paparo GF, Varoli O, Paganelli GM, Labate AM, Masci C. Helicobacter pylori infection and gastric function in patients with chronic idiopathic dyspepsia. Gastroenterology. 1992;103:768-774. [PubMed] |
| 49. | Rhee PL, Kim YH, Son HJ, Kim JJ, Koh KC, Paik SW, Rhee JC, Choi KW. Lack of association of Helicobacter pylori infection with gastric hypersensitivity or delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 1999;94:3165-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |