Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4233
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: July 21, 2005
AIM: Autologous blood donation (ABD) is mainly used to reduce the use of banked blood. In fact, ABD can be regarded as acute blood loss. Would ABD 2-3 d before operation affect the CVP level and subsequently result in less blood loss during liver resection was to be determined.
METHODS: Eighty-four patients undergoing living donor left hepatectomy were retrospectively divided as group I (GI) and group II (GII) according to have donated 250-300 mL blood 2-3 d before living donor hepatectomy or not. The changes of the intraoperative CVP, surgical blood loss, blood products used and the changes of perioperative hemoglobin (Hb) between groups were analyzed and compared by using Mann-Whitney U test.
RESULTS: The results show that the intraoperative CVP changes between GI (n = 35) and GII (n = 49) up to graft procurement were the same, subsequently the blood loss, but ABD resulted in significantly lower perioperative Hb levels in GI.
CONCLUSION: Since none of the patients required any blood products perioperatively, all the predonated bloods were discarded after the patients were discharged from the hospital. It indicates that ABD in current series had no any beneficial effects, in term of cost, lowering the CVP, blood loss and reduce the use of banked blood products, but resulted in significant lower Hb in perioperative period.
- Citation: Jawan B, Cheng YF, Tseng CC, Chen YS, Wang CC, Huang TL, Eng HL, Liu PP, Chiu KW, Wang SH, Lin CC, Lin TS, Liu YW, Chen CL. Effect of autologous blood donation on the central venous pressure, blood loss and blood transfusion during living donor left hepatectomy. World J Gastroenterol 2005; 11(27): 4233-4236
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4233.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4233
Donor hepatectomy with maximal safety while preserving graft viability and adequacy, in terms of size and function for both recipient and donor, is of principal concern in living donor liver transplantation (LDLT). There are compelling reasons for avoiding surgical blood loss with subsequent blood transfusion[1]. Allogenic blood transfusion cannot only transmit the infectious diseases, but also induce variety of immunologic responses, such as alloimmunization, transfusion-associated graft vs host diseases, and immunosup-pression, which would result in malignant tumor recurrence and increased postoperative infection rate[2]. Preoperative autologous blood donation (ABD) has been successfully applied in reducing the use of allogenic blood transfusion in liver resection[3,4]. It is recommended that the last blood donation should not be collected later than 72 h before surgery, to allow for restoration of intravascular volume[5]. Indeed, ABD can be regarded as acute blood loss. On the other side, low intraoperative central venous pressure (CVP) is associated with less surgical blood loss in hepatectomy surgery[4,6,7]. Would preoperative ABD 2-3 d before living donor hepatectomy, without intravenous (IV) crystalloid replacement of the loss, affect the intraoperative CVP levels, subsequently reduce the blood loss and blood transfusion during living donor hepatectomy is to be determined. Aims of this retrospective study were to compare the intraoperative CVP levels, blood loss, and the requirement of blood transfusion in patients with and without preoperative ABD during living donor hepatectomy.
Only consanguineous relatives up to the fifth degree and lawfully wedded spouses are considered legal live donors in Taiwan. Donor volunteers underwent anthropometric measurements, thorough laboratory analysis, psychosocial evaluation, and detailed imaging studies including Doppler ultrasonography to check the quality of liver parenchyma and patency of blood vessels, and magnetic resonance, venography, arteriography, and cholangiography to check hepatic and portal venous anatomy and hepatic artery and biliary tree branching patterns as previously reported[8]. Donors should be free of the active liver disease and the donor-recipient pair must be blood group identical or compatible.
After obtaining approval from the Ethics Committee of the Department of Health, Taiwan and written informed consent for surgery and anesthesia from the patients, the patients came into operation room without premedication and IV line. After establishment of the IV line, the anesthesia was induced with thiopental and fentanyl. Succinylcholine was used for facilitating the tracheal intubation. The anesthesia was maintained with isoflurane in oxygen-air mixture and atracurium was used as muscle relaxant. All patients were monitored with electrocardiography, arterial line for continuous blood pressure monitoring, CVP, pulse oximetry, end tidal CO2, body temperature and urine output.
IV fluids restriction combined with the use of furosemide were applied to lower the intraoperative CVP levels. The deficit of the insensible fluids loss from no per os intake of the patients was not replaced before graft procurement; the intraoperative fluids were maintained to 2-4 mL/(kg·h) before the liver graft is dissected. If the CVP was higher than 10 cm H2O, furosemide was given; second dose of furosemide was also given if the urine output was lower than 0.5 mL/(kg·h). After the liver graft was procured, IV fluid was increased to approximately 10 mL/(kg·h) until the end of the surgery to replace the cumulative deficits from the procedure. Concerning the surgical procedure and liver parenchymal transection with strict adherence to a meticulous surgical technique without vascular inflow, occlusion to either side of the liver has been previously published[1].
The anesthesia records were retrospectively reviewed. Patients who had donated 250-300 mL blood 2-3 d before operation was grouped in group I (GI), while no blood donation in group II (GII). The grouping was not at random or blinded; ABD was performed without normovolemic replacement of the loss by IV infusion of the crystalloid in the first 37 cases. Since none of those patients required blood transfusion after successful LDLT, the protocol of ABD was stopped. Data such as anesthesia time, CVP levels (hourly), blood loss, blood transfusion, crystalloids, doses of furosemide and urine output were collected, compared and analyzed between groups by using Mann-Whitney U test. All the data were given in mean±SD. Statistical calculations were performed using the SPSS advanced statistics module (SPSS Inc., Chicago, IL, USA). P value < 0.05 was regarded as significant.
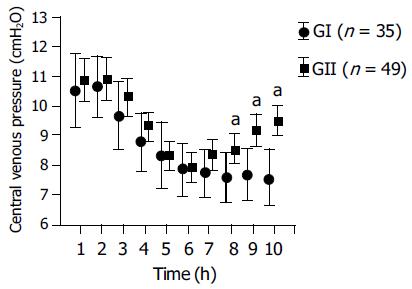
From June 1994 to October 2002, 123 LDLT were performed. Only patients with healthy liver (Table 1) and undergoing left hepatectomy were enrolled in this study. Right hepatectomy (2/37 in GI and 37/86 in GII) was excluded due to most of the patients of ABD received left hepatectomy. Thirty-five patients were included in ABD group (GI) and 49 in non-ABD group (GII). Table 1 shows the characteristics of the patients of GI and GII. The age, weight, liver functions, anesthesia time, blood loss, and mean dose of furosemide used was not significantly different. Likewise, the crystalloids infusion before and after graft procurement was not significantly different either, but the preoperative hemoglobin (Hb) as well as postoperative d 1-4 of GI was significantly lower in GI in comparison to GII. Figure 1 shows the changes of the CVP levels of both groups, the initial CVP of both groups were higher than 10 cm H2O, it decreased gradually, under the influence of fluids restriction and diuresis effect of furosemide, and reached a level of 7.9 ± 2.5, 7.7 ± 2.2 and 7.8 ± 1.7, 8.3 ± 1.8 cm H2O at 6th-7th h after anesthesia begin for GI and GII respectively. It was at that time that the parenchymal transection was performed. After procurement of the liver graft, the IV infusion rate was increased from 3.0 ± 2.3 and 3.4 ± 0.9 to 9.4 ± 6.3 and 10 ± 3.7 mL/(kg·h) for GI and GII respectively. The recovery of the CVP of GI was significantly slower after graft procurement in comparison to GII.
| GI (n = 35) | GII (n = 49) | |
| Age (yr) | 31.1 ± 5.9 | 33.9 ± 8.7 |
| Weight (kg) | 59.1 ± 9.1 | 56 ± 8.8 |
| Gender (female/male) | 24/11 | 31/18 |
| SGOT (U/L) | 17 ± 6.5 | 46.3 ± 4.7 |
| SGPT (U/L) | 15 ± 11.2 | 20.9 ± 4.4 |
| ALP (U/L) | 53.9 ± 21 | 56.4 ± 15.7 |
| Total bilirubin (mg, %) | 0.58 ± 0.22 | 0.67 ± 0.2 |
| BUN (mg, %) | 12.8 ± 3.3 | 15.8 ± 13.9 |
| Creatinine (mg, %) | 0.79 ± 0.7 | 0.75 ± 0.1 |
| Anesthesia time (h) | 10.4 ± 1.5 | 10. ± 1.1 |
| Blood loss (mL) | 63.8 ± 56 | 67 ± 63 |
| Fluid 1 [mL/(kg·h)] | 3.0 ± 2.3 | 3.4 ± 0.9 |
| Fluid 2 [mL/(kg·h)] | 9.4 ± 6.3 | 10 ± 3.7 |
| Urine 1 [mL/(kg·h)] | 1.6 ± 0.7 | 1.6 ± 0.7 |
| Urine 2 [mL/(kg·h)] | 2.0 ± 1.8 | 1.7 ± 1.3 |
| Preoperative Hb (g/dL) | 11.9 ± 1.8a | 12.3 ± 1.5 |
| Postoperative d 1 Hb (g/dL) | 11.4 ± 1.83a | 12.4 ± 1.6 |
| Postoperative d 2 Hb (g/dL) | 11.2 ± 2.0a | 12.3 ± 1.5 |
| Postoperative d 3 Hb (g/dL) | 10.5 ± 1.6a | 11.7 ± 1.4 |
| Postoperative d 4 Hb (g/dL) | 9.8 ± 0.9a | 11.7 ± 1.69 |
| Furosemide (mg) | 11.6 ± 5.0 | 12.2 ± 1.5 |
LDLT is a new form of therapy for pediatric and adult patients with end-stage liver diseases to overcome the problem of organ donor shortage. However, the major medical and ethical concern of this technique is the risk to the healthy donor. This concern is legitimate, since hepatectomy is a major upper abdominal surgery with potential risk of massive blood loss and subsequent requirement for blood transfusion, which is correlated significantly with postoperative morbidity and mortality[9]. It is known that low CVP resulted in significant less blood loss during liver resection. Therefore, maintaining a low CVP has been regarded as a simple and effective way to reduce blood loss during parenchymal transaction[3,4,6]. A low CVP is accompanied by low pressure in the hepatic veins and sinusoids, theoretically favoring less blood loss during parenchymal transection and allows easier control of inadvertent venous injury[1]. Low CVP indicates less intravascular volume and ABD is in fact a kind of acute blood loss. If the volume loss is not replaced, theoretically would result in lowering of the intravascular volume, subsequently the CVP and blood loss of liver resection. Our results show that donation of 250-300 mL of blood 2-3 d before donor hepatectomy without IV fluids replacement seemed not to affect the changes of the CVP levels during the surgical procedure in comparison to the group without ABD (Figure 1). The mechanism is probably due to the fact that the volume loss of the donated blood has been fully compensated by the oral intake of the healthy donors within 2-3 d. Figure 1 shows that, under the same anesthesia, same regime in lowering the CVP by fluids restriction and forced diuresis with furosemide, the changes of the CVP, from the first measurement to graft procurement, were not significantly different between GI and GII. The CVP of both groups decreased gradually from 10.5 ± 3.6 and 10.8 ± 2.8 to 7.9 ± 2.5, 7.7 ± 2.2 and 7.8 ± 1.7, 8.3 ± 1.8 cm H2O at the time of parenchymal transection (around T6-7) for GI and GII respectively. The recovery of the CVP level after procurement of the liver graft to the end of the operation was slower in GI after increasing the infusion rate aimed to replace the cumulative fluid deficit to expand intravascular volume and to preserve renal function. Table 1 shows that the blood loss was not significantly different between groups. Both had only minimal blood loss with a mean loss of 63.8 ± 56 and 67 ± 63 mL for GI and GII respectively.
Current results indicated that ABD itself did not have favorable effect in lowering the intraoperative CVP levels and likewise the blood loss in comparison to non-ABD group. In contrary, it significantly lowered the preoperative Hb level as well as its levels at postoperative d 1-4 (Table 1). It indicated that GI patients would reach earlier low Hb and transfusion threshold. Heiss et al., reported that the blood transfusion rate was indeed higher in the autologous blood group than in the homologous group in patients undergoing colorectal cancer surgery, mainly because these patients had lower preoperative Hb concentrations owing to the blood donation[10].
Allogenic blood transfusion is often life saving and crucial in the treatment of major blood loss[11]. Although the safety of the banked blood supply has been improved recently but it can still have adverse effects[2,12]. The risks of autologous transfusion are less than those of allogenic RBC transfusions[12,13], but it is still not risk free[13-16]. Complications such as bacterial contamination, hemolysis due to administrative error, volume overload were reported[14]. Furthermore, studies have unexpectedly shown similar postoperative infectious complications and immunosup-pression in patients receiving autologous blood donated before surgery, as with those receiving homologous blood[13,17]. Factors such as histamine, plasminogen activator, superoxide and eosinophil cation protein, released into the plasma during storage may be of major importance in enhancing overall immunosuppression[18]. The best policy in avoiding blood transfusion is to improve the surgical and anesthetic technique to minimize blood loss. Anyway, ABD is still an acceptable alternative measurement to reduce the use of allogenic blood if the amount of expected surgical blood loss is large enough requiring blood transfusion[3,4]. Since none of our patients required any blood products perioperatively, the predonated autologous bloods of GI were discarded after the patients being discharged from the hospital. In contrary to previous reports[3,4], ABD in current series had no any beneficial effect, in terms of cost, lowering the CVP, blood loss and reducing the use of banked blood products, but resulted in significant lower Hb in perioperative period, subsequently increasing the chance of getting autologous blood transfusion from their anemia.
In conclusion. Autologous donation of 250-300 mL blood 2-3 d before liver hepatectomy in LDLT seemed not to affect the intraoperative CVP level and subsequently no favorable effect in reducing the blood loss during liver resection. In contrary, it resulted in significant lower Hb in perioperative period, subsequent increasing the chance of getting autologous blood transfusion from their anemia. ABD in living donor left hepatectomy is not recommended if minimal surgical bleeding can be maintained.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Chen CL, Chen YS, de Villa VH, Wang CC, Lin CL, Goto S, Wang SH, Cheng YF, Huang TL, Jawan B. Minimal blood loss living donor hepatectomy. Transplantation. 2000;69:2580-2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Klein HG. Immunomodulatory aspects of transfusion: a once and future risk? Anesthesiology. 1999;91:861-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Chan AC, Blumgart LH, Wuest DL, Melendez JA, Fong Y. Use of preoperative autologous blood donation in liver resections for colorectal metastases. Am J Surg. 1998;175:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Itamoto T, Katayama K, Nakahara H, Tashiro H, Asahara T. Autologous blood storage before hepatectomy for hepatocellular carcinoma with underlying liver disease. Br J Surg. 2003;90:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Vanderlinde ES, Heal JM, Blumberg N. Autologous transfusion. BMJ. 2002;324:772-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 299] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major he-patic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620-625. [RCA] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 367] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Chen YS, Cheng YF, De Villa VH, Wang CC, Lin CC, Huang TL, Jawan B, Chen CL. Evaluation of living liver donors. Transplantation. 2003;75:S16-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Gozzetti G, Mazziotti A, Grazi GL, Jovine E, Gallucci A, Gruttadauria S, Frena A, Morganti M, Ercolani G, Masetti M. Liver resection without blood transfusion. Br J Surg. 1995;82:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Heiss MM, Mempel W, Jauch KW, Delanoff C, Mayer G, Mempel M, Eissner HJ, Schildberg FW. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery. Lancet. 1993;342:1328-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 240] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Greenburg AG. Benefits and risks of blood transfusion in surgical patients. World J Surg. 1996;20:1189-1193. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. First of two parts--blood transfusion. N Engl J Med. 1999;340:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 595] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 13. | Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. Second of two parts--blood conservation. N Engl J Med. 1999;340:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 204] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Linden JV, Kruskall MS. Autologous blood: always safer? Transfusion. 1997;37:455-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Domen RE. Adverse reactions associated with autologous blood transfusion: evaluation and incidence at a large academic hospital. Transfusion. 1998;38:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Goldman M, Rémy-Prince S, Trépanier A, Décary F. Autologous donation error rates in Canada. Transfusion. 1997;37:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Nielsen HJ. Influence on the immune system of homologous blood transfusion and autologous blood donation: impact on the routine clinical practice/differences in oncological and non-tumour surgery? Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35:642-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Nielsen HJ. Detrimental effects of perioperative blood transfusion. Br J Surg. 1995;82:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 150] [Article Influence: 5.0] [Reference Citation Analysis (0)] |