Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4117
Revised: January 1, 2004
Accepted: January 5, 2004
Published online: July 14, 2005
We report a case of the rare solid-pseudopapillary tumor of the pancreas. In contrast to other pancreatic tumors, the solid-pseudopapillary tumor has a favorable prognosis. The 60-year-old female patient we report on here was treated by left pancreatic resection combined with splenectomy for a non-metastasizing tumor of the pancreas. A solid-pseudopapillary tumor was found on histology. The patient had no signs of metastases at present. Since a microscopically invasive tumor growth is assumed, oncologically curative resection should be preferred vs the less radical enucleation. The rare solid-pseudopapillary tumor of the pancreas has a good prognosis after successful oncological resection.
- Citation: Eder F, Schulz HU, Röcken C, Lippert H. Solid-pseudopapillary tumor of the pancreatic tail. World J Gastroenterol 2005; 11(26): 4117-4119
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4117.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4117
Solid-pseudopapillary tumor of the pancreas is a rare condition. In the literature, about 300 cases have been reported. This tumor was first described in 1959[1]. It is also known under the term FRANTZ tumor, named after the author who first described this tumor, which has also been referred to as solid cystic tumor; papillary epithelial neoplasia; solid and papillary epithelial neoplasia; or papillary epithelial tumor. In 1996, this tumor was included in the WHO classification of pancreatic tumors[2]. The origin of the solid-pseudopapillary tumor has not yet been clarified. It is discussed to originate either from ductal epithelium3-6], acinar cells[7-11], or from endocrine cells[12,13]. Another hypothesis is that this tumor arises from pluripotent embryonic cells of the pancreas[12,14,15] or from ridges/ovarian anlage related cells, which were attached to the pancreatic tissue during early embryogenesis[16,17].
Solid-pseudopapillary tumor of the pancreas has a tendency to predominantly affect young women aged between 25 and 35 years[4,8,14,18-22]. Age is reported to range from 8[15] to 70 years[3]. However, a relationship to oral contraception has not been proven[8]. This tumor rarely affects men[23-26] and is characterized by a long asymptomatic course and unspecific symptoms. Therefore, it is not uncommon that solid-pseudopapillary tumor is detected only when it has reached a remarkable size (8-10 cm)[4,10]. Even a tumor size of 20 cm in diameter has been reported[27].
One feature of this tumor is its low malignant potential. Although the liver is found to be the site mostly affected by metastases, these are only rarely seen[15,23,27,28]. Furthermore, there are only few reports about invasive growth[3,4]. Survival time has been reported to reach up to 21 years[1].
Here, we report on a 60-year-old woman, who was found to suffer from a non-metastazing solid-pseudopapillary tumor of the pancreatic tail. Curative resection was perfo-rmed.
A 60-year-old Caucasian woman attended her GP for recurring headache. An X-ray of the spine showed calcifications projecting onto the left upper kidney. Ultrasound and CT-scan of the abdomen showed a polycystic tumor in the tail of the pancreas, which measured approximately 14 cm in maximum diameter.
After the patient was referred to a general hospital a biopsy for cytology was obtained using endoscopic ultrasonography and provided suspicion of a malignant tumor. MRT of the abdomen showed a lesion, which was incompatible with a benign tumor or pseudocyst, and raised suspicion of a cystadenocarcinoma of the pancreas. Finally the patient was referred to our University. The past medical history of the patient included uncomplicated deliveries of four children, no abortions or miscarriages. No history was given suggestive of chronic pancreatitis. The family and social history gave no evidence of a familial cancer syndrome. On admission the patient was mobile, orientated in time, place and person, afebrile and normotonic. Palpation of the abdomen revealed a mild dull pain in the left upper abdomen.
Blood tests showed mild hypercholesterolemia and normal LFT, U&E, and FBC. The tumormarkers CEA, CA19-9 and CA125 were normal. X-ray of the chest and ECG were normal.
A laparotomy was performed and the a tumor of the pancreatic tail explored. No metastases were found. The preparation of the tumor showed a brown colored and good separated growth. A distal pancreatectomy combined with splenectomy was indicated and deperformed. The pancreatic resection margin was free of cancer as demonstrated by frozen section.
Ten days later the patient was discharged from our University hospital without any postoperative complications. The patient is followed up in our tumor dispensaire.
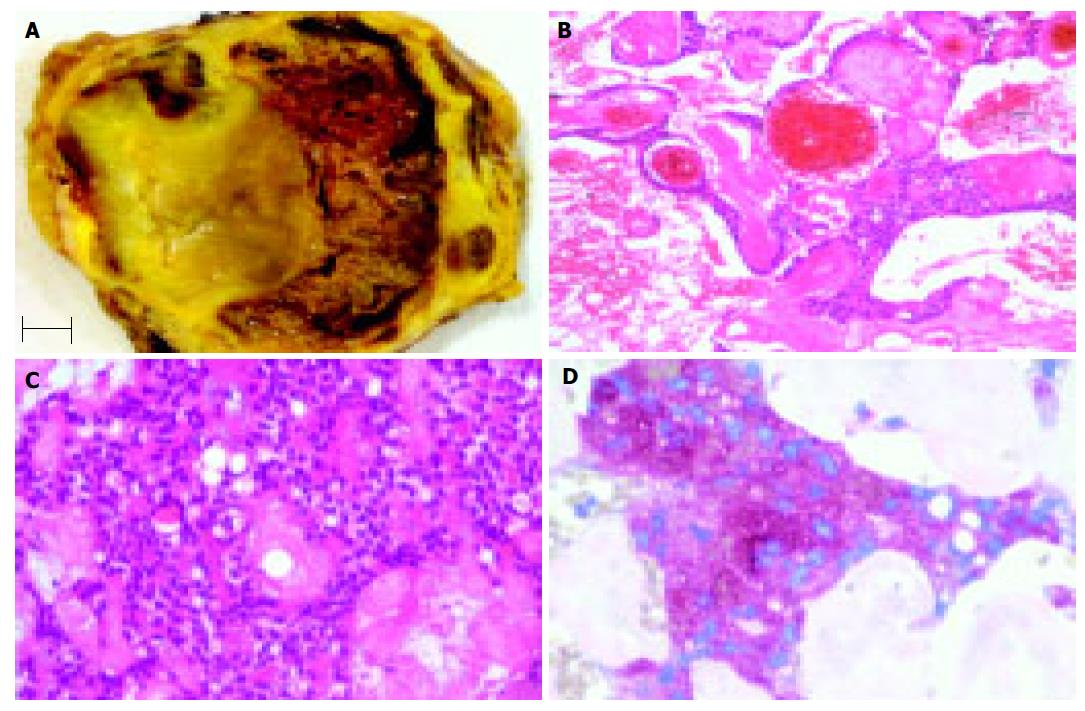
The pancreatic tumor measured 14 cm in greatest diameter and was located in the pancreatic tail. The cut surface was yellow in color with hemorrhages (Figure 1A).
Histologically, the tumor was encapsulated by a fibrous pseudocapsule and characterized by extensive old necroses, large hyalin scars, cholesterin crystals surrounded by foreign body giant cells and calcifications. After extensive sampling small areas of preserved tumor were found under the fibrous capsule. The tumor tissue exhibits a solid monomorphic pattern with small polyhedral cells lining fibrovascular stalks (Figure 1C). A pseudopapillary pattern was noted in areas with regressive changes (Figure 1B). Perineural invasion, angioinvasion, or deep invasion were not found. Immuno-histochemistry with antibodies directed against alpha-1-antitrypsin (Figure 1D), neuron-specific enolase, and CEA showed immunostaining of tumor cells. Immunostaining was not observed with antibodies directed against pancytokeratin (AE1/AE3), CK 8, CK18, chromogranin A, synaptophysin, S-100, and alpha-fetoprotein.
The histological and immunohistochemical findings were in keeping with the diagnosis of a solid-pseudopapillary tumor of the pancreas.
Although the vast majority of patients with a solid pseudo-papillary tumor of the pancreas are young women, our patient, affected by this lesion, was already 60 years old. In 85-90% of the patients the prognosis of the tumor is excellent[16]. Some authors[15,21] described an aggressive growth of the tumor in older patients. Our observations are not in accordance with them. A 53-year-old woman with a solid-pseudopapillary tumor of the pancreatic head treated by radical partial pancreaticoduodenectomy in 1998 is still tumor free at present.
Owing to the slow growth of solid-pseudopapillary tumors, typical early symptoms are lacking. These are only found in conjunction with a higher number of local displacements. In our case, the patient consulted her doctor because of recurrent headache. She never had suffered from epigastric or abdominal pain. In order to investigate putative causes for recurrent headache, X-rays were taken from the spine and calcifications were noted projecting onto the left upper kidney. A tumor of the pancreatic tail was found on ultrasound and CT-scan of the abdomen. In general, owing to the lack of symptoms, a diagnosis of these tumors is possible only when their diameter is in the range of 12-14 cm. There seems to be no correlation between tumor size and behavior[23]. Venous invasion, degree of nuclear atypia, mitotic count and large necrotic clusters are considered histological markers of an increased potential of malignancy[23]. A further criterion is the nuclear DNA-ploidy grade. Kamei and Nishihara[23] demonstrated aneuploidy in patients with metastases, whereas patients without any sign of malignancy showed diploidy. However, this issue is controversially discussed in the literature. In any case, patients exhibiting these markers should be subject to particularly critical surveillance. Opposite to our patient, a complete fibrous capsule always be found in these tumors[23]. Capsular invasions have been described[3] and typically the preserved tumor tissue is found in the periphery, as shown here. Furthermore, tumors may infiltrate into both the sane pancreatic parenchyma and peripancreatic adipose tissue[19]. Therefore, resection according to oncological criteria should be preferred over “radical enucleation”[11] and/or local excision[20]. In addition, since the tumor is confined to the organ or develops metastases only at later time points, radical surgery of a solid-pseudopapillary tumor of the pancreas could be curative.
In conclusion, the patient bearing a solid-pseudopapillary tumor of the pancreas has a good prognosis if the tumor is resected completely, shows no histological evidence of malignancy and demonstrates a diploid population of tumor cells in DNA analysis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Frantz VK. Tumor of the pancreas. In: Blumberg CW (ed) Atlas of Tumor Pathology. Series 1, Fascicles 27 and 28. Washington, DC 1959; 32-33. |
| 2. | Klöppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Histological typing of tumours of the exocrinic pancreas. In: WHO International Classification of Tumours. Berlin Heidelberg New York, Springer 1996; 8452/1. |
| 3. | Compagno J, Oertel JE, Krezmar M. Solid and papillary neo-plasm of the pancreas, probably of small duct origin: A clini-copathologic study of 52 cases. Lab Invest. 1979;40:248-249. |
| 4. | Cubilla AL, Fitzgerald PJ. Tumors of the exocrine pancreas. In: Hartmann WH, Sobin LH (eds.) Atlas of Tumor Pathology; Series 2, Fascicle 19. Washington, DC 1984; 201-207. |
| 5. | Kaufman SL, Reddick RL, Stiegel M, Wild RE, Thomas CG. Papillary cystic neoplasm of the pancreas: a curable pancreatic tumor. World J Surg. 1986;10:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Sanfey H, Mendelsohn G, Cameron JL. Solid and papillary neoplasm of the pancreas. A potentially curable surgical lesion. Ann Surg. 1983;197:272-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Klöppel G, Morohoshi T, John HD, Oehmichen W, Opitz K, Angelkort A, Lietz H, Rückert K. Solid and cystic acinar cell tumour of the pancreas. A tumour in young women with favourable prognosis. Virchows Arch A Pathol Anat Histol. 1981;392:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Learmonth GM, Price SK, Visser AE, Emms M. Papillary and cystic neoplasm of the pancreas--an acinar cell tumour? Histopathology. 1985;9:63-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Morohoshi T, Held G, Klöppel G. Exocrine pancreatic tumours and their histological classification. A study based on 167 autopsy and 97 surgical cases. Histopathology. 1983;7:645-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 163] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Rückert K, Klöppel G, Treu HA, Altmeier A, Hempel D, Lingg G. [Solid-cystic acinar cell tumour of the pancreas (author's transl)]. Dtsch Med Wochenschr. 1982;107:1015-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Schlosnagle DC, Campbell WG. The papillary and solid neoplasm of the pancreas: a report of two cases with electron microscopy, one containing neurosecretory granules. Cancer. 1981;47:2603-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Yagihashi S, Sato I, Kaimori M, Matsumoto J, Nagai K. Papillary and cystic tumor of the pancreas. Two cases indistinguishable from islet cell tumor. Cancer. 1988;61:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Mao C, Guvendi M, Domenico DR, Kim K, Thomford NR, Howard JM. Papillary cystic and solid tumors of the pancreas: a pancreatic embryonic tumor? Studies of three cases and cumulative review of the world's literature. Surgery. 1995;118:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 189] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Matsunou H, Konishi F. Papillary-cystic neoplasm of the pancreas. A clinicopathologic study concerning the tumor aging and malignancy of nine cases. Cancer. 1990;65:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Klöppel G, Kosmahl M. Cystic lesions and neoplasms of the pancreas. The features are becoming clearer. Pancreatology. 2001;1:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Rebhandl W, Felberbauer FX, Puig S, Paya K, Hochschorner S, Barlan M, Horcher E. Solid-pseudopapillary tumor of the pancreas (Frantz tumor) in children: report of four cases and review of the literature. J Surg Oncol. 2001;76:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Rustin RB, Broughan TA, Hermann RE, Grundfest-Broniatowski SF, Petras RE, Hart WR. Papillary cystic epithelial neoplasms of the pancreas. A clinical study of four cases. Arch Surg. 1986;121:1073-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Siech M, Merkle E, Mattfeldt T, Widmaier U, Brambs HJ, Beger HG. Solid pseudopapillary tumors of the pancreas. Chirurg. 1996;67:1012-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Tarpila E, Borch K, Franzen L, Andersson R, Evander A, Lasson A, Lindstr�m CG, Ihse I. Cystic neoplasms of the pancreas: A clinicopathological study of 38 cases. Dig Surg. 1989;6:138-141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Warshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432-43; discussion 444-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 387] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Yamaguchi K, Hirakata R, Kitamura K. Papillary cystic neoplasm of the pancreas: radiological and pathological characteristics in 11 cases. Br J Surg. 1990;77:1000-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Nishihara K, Nagoshi M, Tsuneyoshi M, Yamaguchi K, Hayashi I. Papillary cystic tumors of the pancreas. Assessment of their malignant potential. Cancer. 1993;71:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Ohashi K, Nakajima Y, Hisanaga M, Nakano H, Tsutsumi M, Kondoh S, Konishi Y. A solid and papillary (solid-cystic) tumor of the pancreas occurring in a 36-year-old man: report of a case. Surg Today. 1993;23:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Ohiwa K, Igarashi M, Nagasue N, Nagasaki M, Harada T. Solid and cystic tumor (SCT) of the pancreas in an adult man. HPB Surg. 1997;10:315-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Wilson MB, Adams DB, Garen PD, Gansler TS. Aspiration cytologic, ultrastructural, and DNA cytometric findings of solid and papillary tumor of the pancreas. Cancer. 1992;69:2235-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Cappellari JO, Geisinger KR, Albertson DA, Wolfman NT, Kute TE. Malignant papillary cystic tumor of the pancreas. Cancer. 1990;66:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Horisawa M, Niinomi N, Sato T, Yokoi S, Oda K, Ichikawa M, Hayakawa S. Frantz's tumor (solid and cystic tumor of the pancreas) with liver metastasis: successful treatment and long-term follow-up. J Pediatr Surg. 1995;30:724-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |