Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4108
Revised: November 23, 2004
Accepted: November 26, 2004
Published online: July 14, 2005
AIM: To evaluate the association between Chlamydia pneumoniae (Cpn) infection and primary biliary cirrhosis (PBC).
METHODS: Cpn IgG and IgM were determined by enzyme-linked immunosorbent assay (ELISA) in 41 well-established PBC patients and two race-matched control groups (post-hepatitis cirrhosis, n = 70; healthy controls, n = 57).
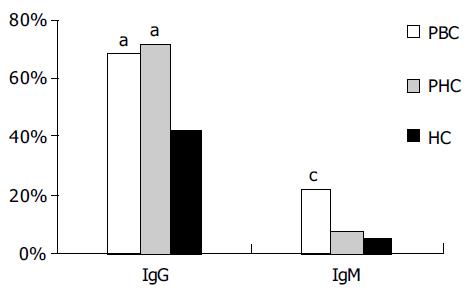
RESULTS: The mean level and seroprevalence of Cpn IgG in PBC group and post-hepatitis cirrhosis (PHC) group were significantly higher than those in healthy controls (46.8 ± 43.4 RU/mL, 49.5 ± 45.2 RU/mL vs 28.3 ± 32.7 RU/mL; 68.3%, 71.4%, 42.1%, respectively; P < 0.05). There was a remarkably elevated seroprevalence of Cpn IgM in patients with PBC (22.0%) compared to the PHC and healthy control (HC) groups. For the PBC patients versus the HCs, the odds ratios (ORs) of the presence of Cpn IgG and IgM were 2.7 (95% CI 0.9-6.1) and 5.1 (95% CI 1.4-18.5), respectively. Though there was no correlation in the level of Cpn IgG with total IgG in sera of patients with PBC (r = -0.857, P = 0.344 > 0.05), Cpn IgM was related with the abnormally high concentrations of total IgM in PBC group.
CONCLUSION: The results of this study do not support the hypothesis that infection with Chlamydia pneumoniae may be a triggering agent or even a causative agent in PBC, but suggest that Chlamydia pneumoniae infection probably contributes to the high level of IgM present in most patients with PBC.
- Citation: Liu HY, Deng AM, Zhang J, Zhou Y, Yao DK, Tu XQ, Fan LY, Zhong RQ. Correlation of Chlamydia pneumoniae infection with primary biliary cirrhosis. World J Gastroenterol 2005; 11(26): 4108-4110
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4108.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4108
PBC is a chronic inflammatory process that mainly affects the medium-sized intrahepatic bile ducts, leads to chronic cholestasis, and often develops to cirrhosis and liver failure that requires liver transplantation. This disease is characterized by the presence of anti-mitochondrial autoantibodies and is considered as an autoimmune disease. However, its exact mechanism of pathogenesis and etiology remains elusive. Several studies have demonstrated cross-reactivity at both B-cell and T-cell levels between autoantigen-pyruvate dehydrogenase complex (PDC) and polypeptides derived from the sequences of potential pathogens[1,2]. It is suggested that molecular mimicry between pathogen and self-antigen might result in tolerance breakdown. A good deal of evidence suggests an infectious component in the development of PBC, and to date, some bacteria and viruses have been found to be linked to with the disease[3-5]. Nevertheless, so far no microbiological agent has been confirmed in PBC, though the possibility of a pathophysiological role for some viral or bacterial micro-organisms has not been excluded. It was reported that infection with the respiratory bacterium Cpn might be a triggering agent or even a causative agent in PBC[6]. If that is the case, antibiotic treatment could be effective. We attempted to confirm these findings serologically by designing a case-control study, to assess whether PBC patients had any serologic evidence of Cpn, and to evaluate the correlation of Cpn infection with PBC by testing the levels of serum antibodies IgG and IgM against Cpn in Chinese patients with PBC.
A total of 211 subjects, recruited in 2002 and 2003, were divided into three groups. Patient groups involved 41 Chinese patients with well-established PBC from Eastern China including Zhejiang, Jiangsu and Shanghai, who met the following diagnostic criteria for PBC recommended by the American Association for the Study of Liver Diseases (AASLD)[7]: (1) presence of cholestatic liver disease manifestations including jaundice, fatigue and pruritus with abnormally high levels of cholestatic parameters such as ALP, serum bilirubin and γ-GT; (2) absence of biliary obstruction as confirmed by ultrasonography, computed tomography, or endoscopic cholangiography; (3) serum positive AMA titre ≥ 1:40. Information of each patient was obtained at the first visit. The ratio of male to female in the study was about 1:6(6/35). The average age of the patients was 52.5 years (range: 29-87 years). All patients showed AMA positive and anti-M2 was detectable in 35 PBC patients (85.4%). Two groups of race-matched control individuals with no known cardiac disease were selected on the basis of similar age and sex. A control group consisted of 70 Chinese patients with PHC. The third control group included 57 healthy subjects for estimating the prevalence of Cpn antibodies in the general population.
The sera were tested at dilution of 1:101 for Cpn specific IgG and IgM antibodies with ELISA kits (Euroimmun, Luebeck, Germany), according to the manufacturer’s instructions. The coated antigens in the wells contained all relevant antigens localized in the outer membrane of HEp-2 cells infected with the “CDC/CWL-029” strain of Cpn. The outer membrane is composed of lipopolysaccharide (LPS) and numerous proteins (outer membrane proteins, OMPs). IgG antibodies against Cpn were quantitated with three calibration sera at concentrations of 2, 20, and 200 RU/mL. IgM antibodies were quantified by calculating a certain ratio against the calibration serum. If the ratio ≥ 1, the serum was defined as positive; if the ratios < 1.0, the serum was defined as negative.
Quantitative measurements of IgG and IgM were analyzed on sera of all PBC patients with a Dade-Behring nephelometer. Anti-mitochondrial autoantibodies (AMA) were tested by indirect immunofluorescence (Euroimmun, Luebeck, Germany). Anti-M2 antibody was tested by immunoblotting assay using a humanized M2 trimer as antigen source.
All statistical analyses were performed with SPSS for Windows 10.0. The frequencies of antibodies were processed by χ2 test or Fisher’s exact test. The t-test was used for the comparison of group means. P < 0.05 was considered statistically significant. Odds ratios (ORs) were calculated with exact 95% confidence intervals (CIs).
The mean level of Cpn IgG in PBC and PHC groups (46.8 ± 43.4, 49.5 ± 45.2 RU/mL, respectively) was much higher than that in the HC group ( 28.3 ± 32.7 RU/mL), and there was no statistical difference between the two groups (P < 0.05). According to the cut-off value of 20 RU/mL recommended by the kits, the frequency of Cpn IgG in PBC, PHC, and HC groups was 28/41 (68.3%), 50/70 (71.4%) and 24/57(42.1%), respectively. The positive rate of Cpn IgG in PBC and PHC groups was different from that in HC group (P = 0.02 and 0.001 respectively as shown in Figure 1). In contrast, the proportion of IgM positive samples in the PBC group (22.0%, 9/41) was much higher than that in the control groups (PHC, 7.1%; HC, 5.3%), and this difference was statistically significant (P = 0.023 for PBC vs PHC and 0.013 for PBC vs HC). Compared to the healthy controls, the ORs of the presence of Cpn IgG and IgM antibodies in the PBC patients were 2.7 (95%CI: 0.9-6.1) and 5.1 (95%CI:1.4-18.5) respectively.
Twenty-eight of the forty-one (68.3%) patients had high total IgG and IgM levels and 22 of the 28 PBC patients were Cpn IgG positive (78.6%), but there was no difference in total IgG level between Cpn IgG positive and negative patients (P = 0.275). However, the correlation between Cpn IgG level and sera total IgG was statistically insignificant (r = -0.857, P = 0.344). Nine of the twenty-eight (32.1%) patients with increased IgM level were Cpn IgM positive. Cpn IgM was related to abnormally high level of total IgM. Anti-mitochondrial antibodies were found in all the 41 PBC patients, 35 cases (85.4%) of them were M2 autoantibodies positive. Between Cpn IgM positive and negative PBC patients, no significant differences were found in age, age at onset, disease duration and other laboratory parameters including ALP, γGT, serum bilirubin and bile acids.
Cpn is a common cause of community acquired acute respiratory infection with a seroprevalence rate of over 50% adults in many countries. It has been shown that Cpn may play a potential role in autoimmune diseases such as artherosclerosis[8], multiple sclerosis[9] and even primary sclerosing cholangitis[10] that is also an autoimmune cholangitis. The presence of Cpn antigen and RNA in biopsies from patients with autoimmune diseases suggests that Cpn antigen may trigger an immune response through molecular mimicry[11,12]. The role of Cpn in the etiology of autoimmunity is however controversial.
It was found that in periportal and lobular hepatocytes of liver tissues from patients with PBC, Cpn antigens are present instead of C.trachomatis by immunohistochemical staining[6], suggesting that Cpn infection is closely related with PBC. High level of Cpn IgG is an important marker for previous infection whereas IgM reactivity represents acute infection. In this study, up to 68.3% PBC patients were infected with Cpn. Though their prevalence and mean level of Cpn IgG were higher than healthy controls, no difference was seen compared to PHC patients. Therefore, it is difficult to conclude that Cpn infection is the cause of PBC or the result of chronic liver diseases.
Abnormally high level of serum IgM is one of the serological characteristics of PBC. However, the reasons for high level of IgM and the nature of IgM remain unclear. In our study, 70% PBC patients had a high level of IgM and about one third of them were Cpn IgM positive, indicating that increased serum IgM in PBC patients is closely related to Cpn IgM and Cpn infection may be one of the factors for abnormally high level of IgM in PBC patients.
Recently, studies with animal model of experimental allergic encephalitis (EAE) indicate that Cpn infection increases the severity of EAE that can be attenuated with anti-infective therapy, and that infection of the central nervous system with Cpn can amplify the autoreactive pool of lymphocytes and regulate the expression of autoimmune diseases[13,14]. Though we did not find any difference between patients with or without acute Cpn infection in clinical features and biochemical parameters, including ALP, AST and GGT, Cpn may secret high immunogenic substrates or provide an inflammatory microenvironment to enhance the self-reactivity of pathogenic T lymphocytes. How Cpn infection acts on the process of PBC remains to be clarified in future studies.
In conclusion, the results of this study do not support the hypothesis that infection with Cpn may be a triggering agent or even a causative agent at least in Chinese patients with PBC, but suggest that Cpn infection may contribute to the high level of IgM present in most PBC patients.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Bogdanos DP, Baum H, Grasso A, Okamoto M, Butler P, Ma Y, Rigopoulou E, Montalto P, Davies ET, Burroughs AK. Microbial mimics are major targets of crossreactivity with human pyruvate dehydrogenase in primary biliary cirrhosis. J Hepatol. 2004;40:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Vilagut L, Vila J, Viñas O, Parés A, Ginés A, Jiménez de Anta MT, Rodés J. Cross-reactivity of anti-Mycobacterium gordonae antibodies with the major mitochondrial autoantigens in primary biliary cirrhosis. J Hepatol. 1994;21:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Butler P, Valle F, Hamilton-Miller JM, Brumfitt W, Baum H, Burroughs AK. M2 mitochondrial antibodies and urinary rough mutant bacteria in patients with primary biliary cirrhosis and in patients with recurrent bacteriuria. J Hepatol. 1993;17:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Mayo I, Arizti P, Parés A, Oliva J, Doforno RA, de Sagarra MR, Rodés J, Castaño JG. Antibodies against the COOH-terminal region of E. coli ClpP protease in patients with primary biliary cirrhosis. J Hepatol. 2000;33:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Xu L, Shen Z, Guo L, Fodera B, Keogh A, Joplin R, O'Donnell B, Aitken J, Carman W, Neuberger J. Does a betaretrovirus infection trigger primary biliary cirrhosis? Proc Natl Acad Sci USA. 2003;100:8454-8459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Abdulkarim AS, Petrovic LM, Kim WR, Angulo P, Lloyd RV, Lindor KD. Primary biliary cirrhosis: an infectious disease caused by Chlamydia pneumoniae? J Hepatol. 2004;40:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 275] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 8. | Shoenfeld Y, Sherer Y, Harats D. Artherosclerosis as an infectious, inflammatory and autoimmune disease. Trends Immunol. 2001;22:293-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Layh-Schmitt G, Bendl C, Hildt U, Dong-Si T, Jüttler E, Schnitzler P, Grond-Ginsbach C, Grau AJ. Evidence for infection with Chlamydia pneumoniae in a subgroup of patients with multiple sclerosis. Ann Neurol. 2000;47:652-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Ponsioen CY, Defoer J, Ten Kate FJ, Weverling GJ, Tytgat GN, Pannekoek Y, Wertheim-Dillen PM. A survey of infectious agents as risk factors for primary sclerosing cholangitis: are Chlamydia species involved? Eur J Gastroenterol Hepatol. 2002;14:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Bachmaier K, Neu N, de la Maza LM, Pal S, Hessel A, Penninger JM. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 278] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Conant SB, Swanborg RH. MHC class II peptide flanking residues of exogenous antigens influence recognition by autoreactive T cells. Autoimmun Rev. 2003;2:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Lenz DC, Lu L, Conant SB, Wolf NA, Gerard HC, Whittum-Hudson JA, Hudson AP, Swanborg RH. A Chlamydia pneumoniae-specific peptide induces experimental autoimmune encephalomyelitis in rats. J Immunol. 2001;167:1803-1808. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Du C, Yao SY, Ljunggren-Rose A, Sriram S. Chlamydia pneumoniae infection of the central nervous system worsens experimental allergic encephalitis. J Exp Med. 2002;196:1639-1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |