Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4045
Revised: November 23, 2004
Accepted: November 26, 2004
Published online: July 14, 2005
AIM: To investigate the effects of adeno-associated virus (AAV) mediated expression of human interferon-γ for gene therapy in experimental hepatic fibrosis in vitro and in vivo.
METHODS: We constructed the recombinant AAV encoding human INF-γ (rAAV- INF-γ) and took the primary rat hepatic stellate cells and carbon tetrachloride induced rats as the experimental hepatic fibrosis model in vitro and in vivo. Immunocytochemistry analysis was used to reveal the expression of α-SMA, the marker protein expressed in hepatic stellate cells. The mRNA expression of TGF-β, TIMP-1, and MMP-13 were analyzed by RT-PCR method. In vivo study, the hydroxyproline content in liver and serum AST, ALT were also detected.
RESULTS: in vitro study, AAV vector could mediated efficient expression of human INF-γ, which inhibit the activation of hepatic stellate cells, decrease the expression of α-SMA and mRNA of TIMP-1, TGF-β, with the MMP-13 unchanged. In vivo study, the histological examination revealed that rAAV- INF-γ could inhibit the progression of the hepatic fibrosis. In the rAAV-INF-γ induced group, the hydroxyproline content and serum AST, ALT level were decreased to 177 ± 28 µg/g wet liver, 668.5 ± 140.0, 458.4 ± 123.5 U/L, compare with the fibrosis control group 236 ± 31 µg/g wet liver, 1 019.1 ± 276.3, 770.5 ± 154.3 U/L, respectively (P < 0.01). mRNA expression of TIMP-1 in the rAAV-INF-γ induced rat liver was decreased while no significant change was observed in TGF-β and MMP-13.
CONCLUSION: All these results indicated that rAAV-INF-γ has potential effects for gene therapy of hepatic fibrosis, which could inhibit the progression of hepatic fibrosis.
- Citation: Chen M, Wang GJ, Diao Y, Xu RA, Xie HT, Li XY, Sun JG. Adeno-associated virus mediated interferon-gamma inhibits the progression of hepatic fibrosis in vitro and in vivo. World J Gastroenterol 2005; 11(26): 4045-4051
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4045.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4045
Liver fibrosis is a very common disease in China and other countries, it is a wound-healing response to chronic liver injury, results from viral hepatitis, ethanol and drug abuse, which if persistent can lead to cirrhosis or liver failure. The main feature of the fibrosis is the increased deposition of extracellular matrix (ECM) caused by activated hepatic stellate cells (HSCs, Ito cells or fat-storing cells)[1,2].
In normal liver, HSCs undergo the quiescent state, contain retinoid lipid droplets and synthesize low levels of matrix proteins. But, as a result of liver injury, HSCs are in the process of activation, proliferate and transform to myofibroblast like cells. Activated HSCs are characterized by loss of retinoid, appearance of α-smooth muscle actin (α-SMA) and procollagen I, III, which are known to be the primary source of excess ECM deposited in liver fibrosis[3-5]. Meanwhile, the balance between generation and degradation of ECM is broken, cytokines and proteases secreted by hepatocytes, Kupffer cells and leukocytes make up a complicated pathways enhance the activation of HSCs[6,7]. Some growth factors such as transforming growth factor-β (TGF-β) is enhanced in both availability and activity, stimulate the transcription of procollagen, fibronectin and other ECM genes. Also effect of the matrix metalloproteinase (MMP-13), the key enzyme in the degradation of ECM, is decreased by over expression of its inhibitor, the tissue inhibitors of matrix metalloproteinase (TIMP-1). Furthermore, the accumulation of ECM in turn increase not only the number of HSCs but also their capacity to synthesiz the matrix proteins, which ultimately results in cirrhosis[1,3,5].
The interferons (INFs) are a family of cytokines with several crucial biological functions, such as antiviral and immunomodulatory activities, which have first been used in the treatment of chronic hepatitis. Among the INFs, INF-α and INF-β (INF type I) are widely used to treat hepatitis C virus (HCV) infection in clinics, whereas INF-γ (INF type II) has a 10-100 fold lower specific antiviral activity than INF type I, but 100-10 000 times more immunomodulatory activities[8,9]. Recently, more and more studies have shown that INF-γ could inhibit the proliferation and activation of HSCs, thus prevent the production of ECM and decrease the expression of procollagen and other cytokines[10-12]. However the serum elimination half-life of INF-γ is only 2-3 h[12]. To make the therapeutic concentration in target organ, administration of INF-γ through a subcutaneous route may results in a much higher level of INF-γ in the serum, which could cause the adverse effects of flu-like symptoms, leucopoenia and mental depression[13].
Therefore, vector mediated local INF-γ expression in the liver could expand the clinical use of INF-γ in hepatic fibrosis. In this study, the AAV was introduced as the delivery vector for gene therapy. AAV is a number of small single stranded DNA viruses of the genus Dependovirus in the Parvoviridae family. Not only is AAV non-pathogenic and of minimal immune responses, but it could also infect both dividing and terminally-differentiated cells with high efficiency, expressing the transgene for at least 6 mo. Moreover it has a broad range of host cells and tissues[14-18]. We constructed the recombinant AAV coding human INF-γ and examined its potential effects for gene therapy of hepatic fibrosis in vitro and in vivo.
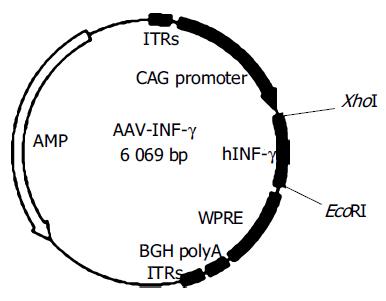
The plasmid of AAV vector, rAAV-eGFP and packaging helper plasmids pAd, pAAD are kindly provided by Prof Rui-An Xu (University of Hong Kong, Genome Research Center). The recombinant plasmid rAAV-INF-γ contains a hybrid of the cytomegalovirus immediate-early enhancer and the chicken β-actin/rabbit β-globin promoter (CAG), inserted with the reporter gene, a 511bp EcoRI/XhoI fragment of complementary DNA (cDNA) encoding full-length human INF-γ. Also the woodchuck hepatitis B virus post-transcriptional regulatory element is inserted to boost expression levers (Figure 1).
To generate the rAAV virions, we cotransfect the recom-binant plasmid AAV-INF-γ or AAV-eGFP and two packaging helper plasmids into HEK 293 cells (ATCC CRL 1573) by calcium phosphate precipitate. The cells were harvested 60 h after transfection and lysed by incubating with 5 g/L deoxycholate in the presence of 50 U/mL benzonase (Sigma, St. Louis, MO, USA), then isolated the recombinant AAV particles by using heparin affinity column chromatography (Amersham, HiTrap). The titles of the rAAV-INF-γ and rAAV-eGFP preparations were determined by AAV Titration ELISA kit (Progen). The virions were stored at -80°C before experimental use.
Primary rat HSCs were extracted from Sprague-Dawley rat (450-550 g, Animal Center of China Pharmaceutical University, Nanjing, China) liver by sequential in situ perfusion with collagenase and pronase followed by density gradient centrifugation and then cultured in 20% FBS DMEM, maintained in a humidified 50 mL/L CO2 incubator at 37°C[19,20]. Experiments described in this study were performed on HSCs between the second and ninth serial passages with highly activated cells as tested. HSCs were cultured in 10 cm culture plates at a density of 2×104 cells/cm2. After changing the medium, HSCs were maintained in presence or absence of rAAV-INF-γ and rAAV-eGFP at 5 × 104 v.g./cell for 72 h, meanwhile collect the medium every 12 h to analysis the expression of human INF-γ by ELISA. Then HSCs were in preparation for RNA isolation and immunocytochemistry analysis.
We used the ELISA kit (R&D systems) to quantitative determine the expression of human INF-γ in the cell culture supernatant. The antibody specific for human INF-γ has been pre-coated onto a microplate. Pipette the samples into the wells, any present INF-γ was bound by the immobilized antibody. Then washed away the unbound substances, an enzyme-liked antibody was added to the wells. After washing any unbound antibody-enzyme reagent, a substrate solution was added to the wells and color developed in proportion to the amount of INF-γ bound in the initial step. Finally, stop the reaction and determined the optical density of each well at 540 or 570 nm within 30 min.
HSCs were washed with PBS and fixed in the slides with 40 g/L phosphate buffered paraformaldehyde (PFA) at 4°C for 20 min. Then eliminated endogenous peroxidase activity by adding 30 mL/L H2O2 in PBS at room temperature for 30 min to permeabilized and blocked with goat serum blocking solution at room temperature for 1 h. After blocking, cells were incubated with primary antibody, anti-α-SMA (diluted 1:200 in PBS, Sigma) at 4°C overnight. Washed the slides thrice in PBS, incubated the cells with secondary antibody biotinylated anti-mouse IgG from goat for 30 min, washed five times and added the solution of avidin-biotin peroxidase. Incubating for 30 min, the cells were then stained with DAB (3,3’-diaminobenzidine tetrahydrochloride, Sigma) peroxidase substrate solution-brown and counterstained with hematoxylin (Sigma). Finally, dehydrate the slides, clear in xylene and coverslip with permanent mounting medium.
Total RNA was extracted from HSCs and snap-frozen liver tissue using TRIzol Reagent (Gibco BRL) according to the method of Chomczynski and Sacchi[21]. Then 4 µg RNA of each sample was used for cDNA synthesis using a RevertAid First Strand cDNA Synthesis Kit (Fermentas). The ampli-fication was carried out with 1 µL of synthesized cDNA in a 50 µL PCR mixture containing 20 pmol of a primer pair, 1.5 mmol/L MgCl2, 0.2 mmol/L dNTPs and 1 U of recombinant Taq DNA polymerase. In total, 30 cycles of the reaction were carried out at 95°C for 1 min, 57°C for 1 min and 72°C for 2 min. The PCR products were electrophoresed on a 20 g/L agarose gel and stained with ethidium bromide. The primers used in this study are listed in Table 1. The density of the band was analyzed using a BIO-RAD Gel Doc 2000 densitometer.
| Gene | Forward primer | Reverse primer |
| INF-γ | 5’-CCGCTCGAGATGAAATATACAAGTTATATC-3’ | 5’-CGGAATTCTTACTGGGATGCTCTTCGACC-3’ |
| TGF-β | 5’-CTTCAGCTCCACAGAGAAGAACTGC-3’ | 5’-CACGATCATGTTGGACAACTGCTCC-3’ |
| TIMP-1 | 5’-CCGCAGACGGCGTTCTGCAA-3’ | 5’-TCGAGACCCAAGGGATTGCC-3’ |
| MMP-13 | 5’-TGACTATGCGTGGCTGGAA-3’ | 5’-AAGCTGAAATCTTGCCTTGGA-3’ |
| GAPDH | 5’-GTCAACGGATTTGGTCGTATT-3’ | 5’-AGTCTTCTGGGTGGCAGTGAT-3’ |
Hepatic fibrosis was induced in male Sprague-Dawley rats (140-150 g) by carbon tetrachloride (CCl4) administration[22]. Animals were maintained on standard chow and water. A total of 48 rats administered 500 mL/L CCl4 dissolved in olive oil at a dose of 1 mL CCl4/kg body weight twice weekly (every Monday and Thursday) via gastrogavage for 8 wk. One group of six rats received olive oil as control. This method reproducibly results in early fibrosis within 3-4 wk, and advanced fibrosis by 6-8 wk[23]. After 3 wk, six rats of CCl4 treated and untreated group were killed for examination of hepatic fibrosis development. After 6 wk, the remaining model rats were injected via the hepatic portal vein with a single infusion of 500 µL of either saline (n = 8), 3 × 1011 v.g./mL rAAV-INF-γ (n = 8), and 3×1011 v.g./mL rAAV-eGFP (n = 8). After 8 wk, all the rats were killed and the liver were fixed in 40 g/L PFA for histological examination or snap-frozen in liquid nitrogen in preparation for measurement of hydroxyproline and RNA isolation. Also the blood samples were taken immediately before the rats were sacrificed for biochemical analysis.
For light-microscopic examination, the fixed liver specimens were embedded in paraffin. Sections were cut at a thickness of 4 µm and stained with hematoxylin-eosin for routine examination.
The liver tissue was finely minced and then homogenized in nine volumes of ice-cold PBS, hydrolyzed in HCl and the hydroxyproline content was determined by the method of Sakaida et al[24] with minor modifications.
Blood was collected from femoral artery, allow the samples to clot for 30 min before centrifugation for 10 min at 1 000 r/min. Remove serum for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) determination.
The data presented are the mean±SD of each group. A statistical analysis was performed using the Student’s t test, P < 0.05 was considered to be statistically significant.
Freshly isolated HSCs could emit blue-green fluorescence, which is easily quenched, stimulated by radiation of 328 nm laser source. Five days after isolation, HSCs exhibited expanded cytoplasmic processes and enlarged nuclei corresponding to the intermediate stage of the activation process. After 10 d culture, HSCs could undergo a totally activated process.
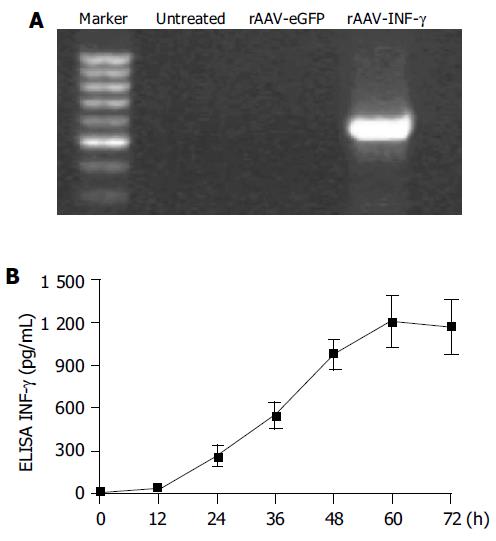
To confirm that AAV-INF-γ was transcriptional active, total RNA of infected HSCs was isolated and examined by RT-PCR analysis. Human INF-γ mRNA transcripts were detected in HSCs infected with rAAV-INF-γ, but undetectable in sham-infected or rAAV-eGFP infected HSCs (Figure 2A). Also the expression and secretion of human INF-γ was next evaluated by ELISA. In the group of rAAV-eGFP infected HSCs, 24 h after infection, human INF-γ expression was 265 ± 67 pg/mL in the supernatant and its level reaches the peak value, 1 211 ± 178 pg/mL at 60 h after infection (Figure 2B).
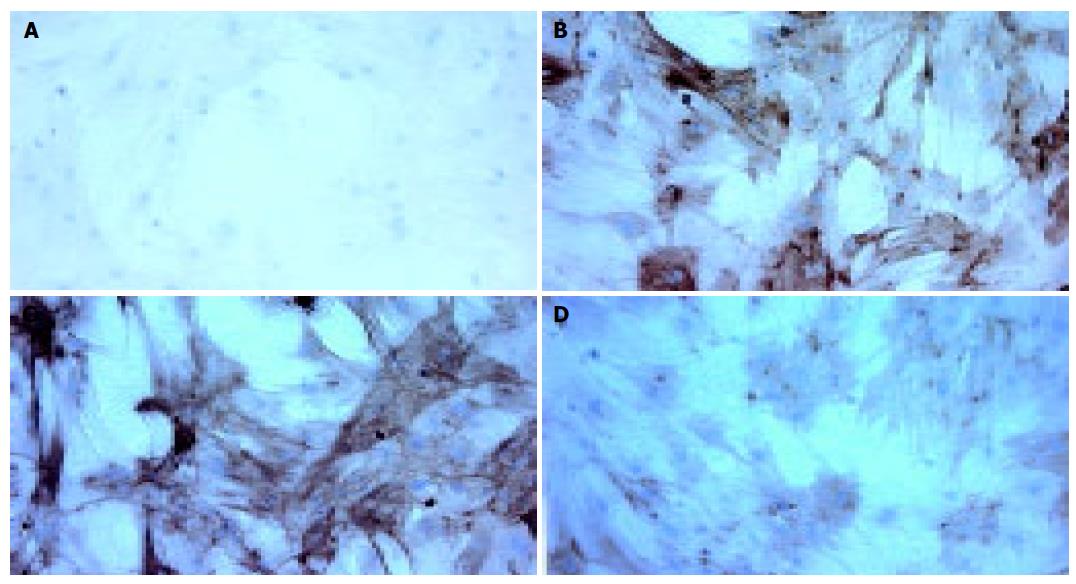
Because α-SMA is the marker protein of activated HSCs, the immunocytochemistry examination was introduced to evaluate the effects of rAAV-INF-γ on activated HSCs. The results revealed that the expression of α-SMA was suppressed by supplementation with rAAV-INF-γ (Figure 3A-D).
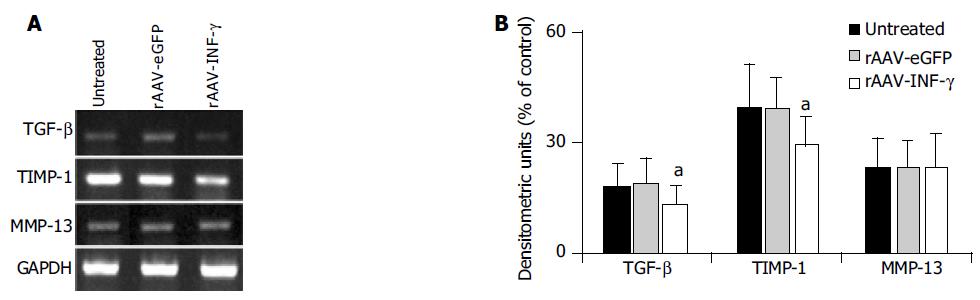
Additionally, INF-γ has a notable feature of immuno-modulatory activity, which could affect the cytokines and proteases expression in HSCs. The mRNA expression of TGF-β, MMP-13 and TIMP-1 between the activated HSCs and HSCs infected with rAAV-eGFP and rAAV-INF-γ were examined by RT-PCR method (Figure 4A). There was no significant difference in the mRNA expression between the untreated and rAAV-eGFP infected HSCs. But the levels of TGF-β and TIMP-1 mRNA were decreased in the rAAV-INF-γ infected HSCs, with an unchanged expression in MMP-13 in contrast to those two groups of HSCs (Figure 4B).
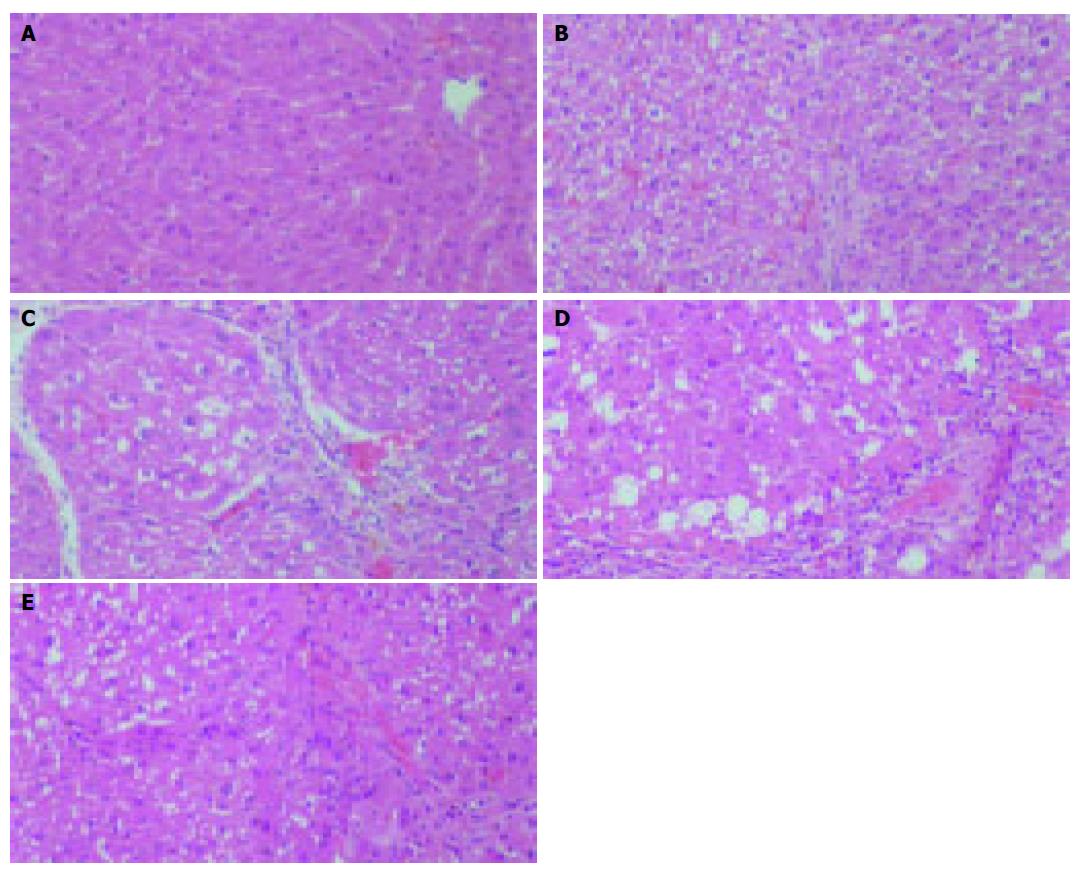
Histological analysis confirmed that CCl4 could cause the hepatic fibrosis. After 3 wk, the mature collagen fibrils bridging vascular structures were obviously presented in the CCl4 treated group, showing a early stage of hepatic fibrosis (Figures 5A and B). At the end of 8 wk, the saline and rAAV-eGFP treated model group revealed cirrhotic-like structural patterns: fibrous connective tissue components in Glisson’s sheath, pseudolobule formations and formation of fibrotic septa and thickened reticulin fibers joining central areas. In contrast, the rat liver that received rAAV-INF-γ showed only light bridging fibrosis and diminished fibrosis in both the periportal and centrilobular liver (Figures 5C-E).
In collagen, 13.4% of total amino acid is hydroxyproline, whereas little content in elastin and inexistence in other proteins. So hydroxyproline content could reflect the collagen content in connective tissue. Determination of hydroxyproline content in different groups of rat liver confirmed the histological studies that hydroxyproline content in the rAAV-INF-γ treated group was significantly reduced to 177 ± 28 µg/g wet liver, compare with the fibrosis control group 236 ± 31 µg/g wet liver (Table 2).
The serum AST and ALT levels in the group of rats treated with CCl4/saline and CCl4/rAAV-eGFP were 1 019.1 ± 276. 3, 770.5 ± 154.3 U/L and 985.4 ± 241.6, 727.2 ± 175.0 U/L, respectively. No significant difference was observed between those two groups. In contrast to the CCl4/saline control group, the serum AST and ALT level of CCl4/rAAV-INF-γ treated rats were reduced to 668.5 ± 140.0, 458.4 ± 123.5 U/L, suggesting that the INF-γ gene expression inhibited the hepatic toxicity caused by CCl4 (Table 2).
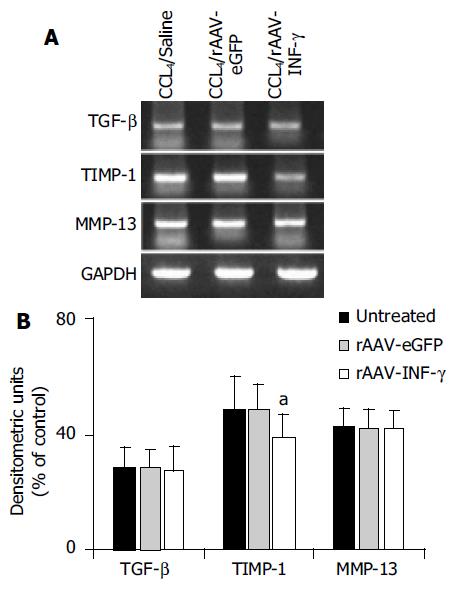
The mRNA expression of TGF-β, MMP-13 and TIMP-1 between the control group and the group induced with rAAV-INF-γ were analyzed by RT-PCR method (Figure 6A). There was a similar result between the in vivo and in vitro study, with one difference that mRNA expression of TGF-β were unchanged in three groups of rat liver (Figure 6B).
Liver fibrosis is reversible, whereas cirrhosis, the end stage consequence of fibrosis, is generally irreversible. Thus, efforts to understand fibrosis may focus primarily on events that lead to the early accumulation of ECM in hopes of identifying therapeutic targets to slow its progression[25,26]. Endogenous INF-γ is a lymphokine produced by natural killer cells and T lymphocytes, which plays a central role in the control of the immune response. It is also known for its regulatory effects on collagen synthesis. Tissue repair is characterized by infiltration of inflammatory, immune and mesenchymal cells in injured areas accompanied by release of cytokines, followed proliferation of mesenchymal cells involved in synthesis and deposition of collagen. The effects of INF-γ on collagen production have been the objects of a number of studies suggest that INF-γ acts as an endogenous mediator reducing collagen synthesis[27,28].
In this study, rAAV-INF-γ could infect the primary cultured HSCs effectively. The expression of INF-γ reached the peak level, 1 211 ± 178 pg/ml, at 60 h after infection with 5 ± 104 v.g./cell rAAV-INF-γ. α-SMA expression and mRNA of TGF-β, TIMP-1 were down regulated with the expression of INF-γ, attenuated the HSCs activation and proliferation in vitro. Also, rAAV-INF-γ could inhibit the development of experimental hepatic fibrosis induced by CCl4 administration in vivo, though it seemed hard to reverse it. The histology results revealed that hepatic fibrosis rats treated with rAAV-INF-γ showed light fibrosis compared with the control groups. In the rAAV-INF-γ induced group, the hydroxyproline content and serum AST, ALT level were decreased to 177 ± 28 µg/g wet liver, 668.5 ± 140.0, 458.4 ± 123.5 U/L, compare with the fibrosis control group 236 ± 31 µg/g wet liver, 1 019.1 ± 276.3, 770.5 ± 154.3 U/L, respectively (P < 0.01). mRNA expression of TIMP-1 in the rAAV-INF-γ induced rat liver was decreased while no significant change was observed in TGF-β and MMP-13.
There is a difference between in vitro and in vivo study, expression of TGF-β mRNA was inhibited in HSCs infected with rAAV-INF-γ, but keeps unchanged in AAV-INF-γ treated rat liver compare with the control group. TGF-β is the important mediator involved in the transdifferentiation of HSCs from quiescent to activated phenotypes and biosynthesis of collagens type I, resulting in production of ECM[29]. In liver, Kupffer cells are another main source of TGF-β besides HSCs. Since INF-γ could also activate macrophages including Kupffer cells, the total effect of INF-γ in liver does not change the mRNA expression of TGF-β[30]. Thus the protein production and receptor expression of TGF-β should be investigated to make a more elaborate description of the change about TGF-β modulated by INF-γ.
The majority of clinical use of INF-γ up to date has been conducted with subcutaneous route of purified recombinant INF-γ protein. The cost of standard INF-γ regimen is very expensive that limits its access to the patients. So gene therapy appears to be the more powerful strategy than other forms in gene therapy of hepatic fibrosis. Potential advantages of gene therapy are sustained expression of transgene and highly localized delivery within a single injection. The advantages of localized vector delivery are obvious because it can induce a high level of transgenic proteins in situ to achieve potential efficient activity and reduce adverse effects compared with systemic delivery. In resent studies, several gene delivery systems were reported for gene therapy targeting the liver disease. Adenoviruses are the vectors used most commonly for gene therapy, but its effective host immune response and the toxic/anaphylactic reaction limits its clinical use[31-32]. Protzer et al[33] demonstrated that INF gene transfer by the hepatitis B virus vector efficiently suppressed wild-type virus infection, however the gene transfer efficiency in the liver is comparatively low. Compare with those two vectors, AAV vectors have little immunogenicity and far higher transfer efficiency. Furthermore, AAV vectors can mediate the long-term in vivo expression in host cells, with persistent expression up to 6 mo[18,34,35]. Actually, the human clinical trials for cystic fibrosis and for hemophilia B have been enrolling patients since 1996 and 1999, respectively, via recombinant AAV vectors. While comprehensive reviews exist of the current scope of clinical conditions for AAV vectors are being developed, it is likely that clinical trials involving delivery to the liver will commence in the near future[15].
Taking all these results together, rAAV-INF-γ has potential effects for gene therapy of hepatic fibrosis. We believe that this system could now be applied in clinical trials, though we must continue to explore critical pre-clinical evaluation of risks in the age of clinical trials.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Hui AY, Friedman SL. Molecular basis of hepatic fibrosis. Expert Rev Mol Med. 2003;5:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [PubMed] |
| 3. | Safadi R, Friedman SL. Hepatic fibrosis--role of hepatic stellate cell activation. MedGenMed. 2002;4:27. [PubMed] |
| 4. | Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28-36. [PubMed] |
| 5. | Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol. 1997;32:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | McGuire RF, Bissell DM, Boyles J, Roll FJ. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992;15:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 105] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 389] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 998] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 9. | Shen H, Zhang M, Minuk GY, Gong Y. Different effects of rat interferon alpha, beta and gamma on rat hepatic stellate cell proliferation and activation. BMC Cell Biol. 2002;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Sakaida I, Uchida K, Matsumura Y, Okita K. Interferon gamma treatment prevents procollagen gene expression without affecting transforming growth factor-beta1 expression in pig serum-induced rat liver fibrosis in vivo. J Hepatol. 1998;28:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Mallat A, Preaux AM, Blazejewski S, Rosenbaum J, Dhumeaux D, Mavier P. Interferon alfa and gamma inhibit proliferation and collagen synthesis of human Ito cells in culture. Hepatology. 1995;21:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Thompson JA, Cox WW, Lindgren CG, Collins C, Neraas KA, Bonnem EM, Fefer A. Subcutaneous recombinant gamma interferon in cancer patients: toxicity, pharmacokinetics, and immunomodulatory effects. Cancer Immunol Immunother. 1987;25:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Baroni GS, D'Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 204] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today. 2000;6:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 152] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Monahan PE, Samulski RJ. AAV vectors: is clinical success on the horizon? Gene Ther. 2000;7:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 206] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | During MJ, Symes CW, Lawlor PA, Lin J, Dunning J, Fitzsimons HL, Poulsen D, Leone P, Xu R, Dicker BL. An oral vaccine against NMDAR1 with efficacy in experimental stroke and epilepsy. Science. 2000;287:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Cao L, Liu Y, During MJ, Xiao W. High-titer, wild-type free recombinant adeno-associated virus vector production using intron-containing helper plasmids. J Virol. 2000;74:11456-11463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Xu R, Sun X, Tse LY, Li H, Chan PC, Xu S, Xiao W, Kung HF, Krissansen GW, Fan ST. Long-term expression of angiostatin suppresses metastatic liver cancer in mice. Hepatology. 2003;37:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Riccalton-Banks L, Bhandari R, Fry J, Shakesheff KM. A simple method for the simultaneous isolation of stellate cells and hepatocytes from rat liver tissue. Mol Cell Biochem. 2003;248:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 217] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39099] [Article Influence: 1028.9] [Reference Citation Analysis (0)] |
| 22. | Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1183-1190. [PubMed] |
| 23. | Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193-203. [PubMed] |
| 24. | Sakaida I, Hironaka K, Uchida K, Suzuki C, Kayano K, Okita K. Fibrosis accelerates the development of enzyme-altered lesions in the rat liver. Hepatology. 1998;28:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Benyon RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000;46:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 886] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 27. | Czaja MJ, Weiner FR, Eghbali M, Giambrone MA, Eghbali M, Zern MA. Differential effects of gamma-interferon on collagen and fibronectin gene expression. J Biol Chem. 1987;262:13348-13351. [PubMed] |
| 28. | Rockey DC, Maher JJ, Jarnagin WR, Gabbiani G, Friedman SL. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992;16:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Tiggelman AM, Boers W, Linthorst C, Sala M, Chamuleau RA. Collagen synthesis by human liver (myo)fibroblasts in culture: evidence for a regulatory role of IL-1 beta, IL-4, TGF beta and IFN gamma. J Hepatol. 1995;23:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Suzuki K, Aoki K, Ohnami S, Yoshida K, Kazui T, Kato N, Inoue K, Kohara M, Yoshida T. Adenovirus-mediated gene transfer of interferon alpha improves dimethylnitrosamine-induced liver cirrhosis in rat model. Gene Ther. 2003;10:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Abriss B, Hollweg G, Gressner AM, Weiskirchen R. Adenoviral-mediated transfer of p53 or retinoblastoma protein blocks cell proliferation and induces apoptosis in culture-activated hepatic stellate cells. J Hepatol. 2003;38:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Protzer U, Nassal M, Chiang PW, Kirschfink M, Schaller H. Interferon gene transfer by a hepatitis B virus vector efficiently suppresses wild-type virus infection. Proc Natl Acad Sci USA. 1999;96:10818-10823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Ferry N, Heard JM. Liver-directed gene transfer vectors. Hum Gene Ther. 1998;9:1975-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | de Roos WK, Fallaux FJ, Marinelli AW, Lazaris-Karatzas A, von Geusau AB, van der Eb MM, Cramer SJ, Terpstra OT, Hoeben RC. Isolated-organ perfusion for local gene delivery: efficient adenovirus-mediated gene transfer into the liver. Gene Ther. 1997;4:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |